Unnatural C-1 homologues of pancratistatin - Synthesis and promising biological activities
- PMID: 28017970
- PMCID: PMC5176109
- DOI: 10.1139/v2012-073
Unnatural C-1 homologues of pancratistatin - Synthesis and promising biological activities
Abstract
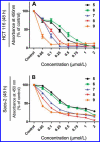
Several C-1 homologues of pancratistatin and 7-deoxypancratistatin were synthesized by a phenanthrene-phenathridone oxidative recyclization strategy. The key steps involved the enzymatic dihydroxylation of bromobenzene, addition of an aryl alane to an epoxyaziridine, an intramolecular aziridine opening on silica gel in solid phase, and the above-mentioned recylization strategy. Experimental and spectral data are reported for all new compounds. All synthesized C-1 homologues of pancratistatin and 7-deoxypancratistatin were evaluated for antiproliferative activity in a panel of human cancer cell lines. As expected, the 7-hydroxy compounds were found to be more potent and the activity of the C-1 benzoxymethyl analogue exceeded that of narciclasine, which was used as a positive control.
On a réalisé la synthèse de plusieurs homologues en C-1 de la pancratistatine et de la 7-désoxypencratistatine en faisant appel à une stratégie de recyclisation oxydante phenantrène–phénathridone. Les étapes clés impliquent la dihydroxylation du bromobenzène, l’addition d’une arylalane à une époxyaziridine, une ouverture intramoléculaire d’aziridine sur gel de silice en phase solide et la stratégie de recyclisation mentionnée plus haut. Les données expérimentales et spectrales sont rapportées pour tous les nouveaux produits. Tous les homologues en C-1 de la pancratistatine et de la 7-désoxypencratistatine ont été évalués pour leur activité à contrer la prolifération dans un éventail de lignées de cellules cancéreuses humaines. Tel que prévu, les composés 7-hydroxy sont les plus puissants alors que l’activité de l’analogue C-1 benzoxyméthyle est supérieure à celle de la narciclasine qui a été utilisée comme contrôle positif.
Keywords: C-1 homologues of pancratistatin; amaryllidaceae alkaloids; anticancer activities; intramolecular aziridine opening; solid-phase silica catalysis.
Figures
References
-
- Pettit GR, Gaddamidi V, Cragg GM, Herald DL, Sagawa Y. J. Chem. Soc. Chem. Commun. 1984;(24):1693. doi:10.1039/c39840001693.
-
- Kornienko A, Evidente A. Chem. Rev. 2008;108(6):1982. For reviews see. doi:10.1021/cr078198u. - PMC - PubMed
- Manpadi M, Kornienko A. Org. Prep. Proced. Int. 2008;40(2):107. doi:10.1080/00304940809458083. - PMC - PubMed
- Chapleur Y, Chrétien F, Ahmed SI, Khaldi M. Curr. Org. Synth. 2006;3(3):341. doi:10.2174/157017906777934872.
- Rinner U, Hudlicky T. Synlett. 2005:365.
- Bridges A. Chemtracts: Organic Chemistry. 1996;9:101.
-
- Collins J, Rinner U, Moser M, Hudlicky T, Ghiviriga I, Romero AE, Kornienko A, Ma D, Griffin C, Pandey S. J.Org. Chem. 2010;75(9):3069. For a recent compilation of total syntheses of Amaryllidaceae constituents see. doi:10.1021/jo1003136. - PMC - PubMed
- Yadav JS, Satheesh G, Murthy CVSR. Org. Lett. 2010;12(11):2544. For syntheses published after 2009 see. doi:10.1021/ol100755v. - PubMed
- Dam JH, Madsen R. Eur. J. Org. Chem. 2009;2009(27):4666. doi:10.1002/ejoc.200900719.
-
- Pettit GR, Melody N, O'Sullivan M, Thompson MA, Herald DL, Coates B. J.Chem. Soc. Chem. Commun. 1994:2725. doi:10.1039/c39940002725.
- Pettit GR, Freeman S, Simpson MJ, Thompson MA, Boyd MR, Williams MD, Pettit GR, III, Doubek DL. Anticancer Drug Des. 1995;10:243. - PubMed
- Pettit GR, Melody N, Herald DL. J. Org. Chem. 2001;66(8):2583. doi:10.1021/jo000710n. - PubMed
- Pettit GR, Melody N, Herald DL, Knight JC, Chapuis J-C. J. Nat. Prod. 2007;70(3):417. doi:10.1021/np068046e. - PubMed
- Pettit GR, Ducki S, Eastham SA, Melody N. J.Nat. Prod. 2009;72(7):1279. doi:10. 1021/np9001948. - PMC - PubMed
- Pettit GR, Tan R, Bao G-H, Melody N, Doubek DL, Gao S, Chapuis J-C, Williams L. J.Nat. Prod. 2012;75(4):771. doi:10.1021/np200862y. - PMC - PubMed
-
- McNulty J, Mao J, Gibe R, Mo R, Wolf S, Pettit GR, Herald DL, Boyd MR. Bioorg. Med. Chem. Lett. 2001;11(2):169. doi:10.1016/S0960-894X(00)00614-4. - PubMed
- McNulty J, Larichev V, Pandey S. Bioorg. Med. Chem. Lett. 2005;15(23):5315. doi:10.1016/j.bmcl.2005.08.024. - PubMed
- McNulty J, Nair JJ, Griffin C, Pandey S. J.Nat. Prod. 2008;71(3):357. doi:10.1021/np0705460. - PubMed
- McNulty J, Nair JJ, Little JRL, Brennan JD, Bastida J. Bioorg. Med. Chem. Lett. 2010;20(17):5290. doi:10.1016/j.bmcl.2010.06.130. - PubMed
- McNulty J, Nair JJ, Singh M, Crankshaw DJ, Holloway AC. Bioorg. Med. Chem. Lett. 2010;20(7):2335. doi:10.1016/j.bmcl.2010.01.157. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials