Positive Allosteric Modulation of Insect Olfactory Receptor Function by ORco Agonists
- PMID: 28018173
- PMCID: PMC5145856
- DOI: 10.3389/fncel.2016.00275
Positive Allosteric Modulation of Insect Olfactory Receptor Function by ORco Agonists
Abstract
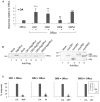
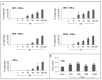
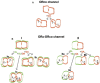
Insect olfactory receptors (ORs) are heteromeric ligand-gated cation channels composed of a common olfactory receptor subunit (ORco) and a variable subunit (ORx) of as yet unknown structures and undetermined stoichiometries. In this study, we examined the allosteric modulation exerted on Anopheles gambiae heteromeric ORx/ORco olfactory receptors in vitro by a specific class of ORco agonists (OAs) comprising ORcoRAM2 and VUAA1. High OA concentrations produced stronger functional responses in cells expressing heteromeric receptor channels relative to cells expressing ORco alone. These OA-induced responses of ORx/ORco channels were also notably much stronger than those obtained upon administration of ORx-specific ligands to the same receptors. Most importantly, small concentrations of OAs were found to act as strong potentiators of ORx/ORco function, increasing dramatically both the efficacy and potency of ORx-specific odorants. These results suggest that insect heteromeric ORs are highly dynamic complexes adopting different conformations that change in a concerted fashion as a result of the interplay between the subunits of the oligomeric assemblies, and that allosteric modulation may constitute an important element in the modulation and fining tuning of olfactory reception function.
Keywords: Anopheles gambiae; ORco agonists; cell-based screening; ligand discovery; malaria; mosquito olfaction; olfactory function enhancement; olfactory receptor pharmacology.
Figures


Similar articles
-
Natural volatiles preventing mosquito biting: An integrated screening platform for accelerated discovery of ORco antagonists.J Biol Chem. 2024 Dec;300(12):107939. doi: 10.1016/j.jbc.2024.107939. Epub 2024 Oct 29. J Biol Chem. 2024. PMID: 39476965 Free PMC article.
-
Allosteric antagonism of insect odorant receptor ion channels.PLoS One. 2012;7(1):e30304. doi: 10.1371/journal.pone.0030304. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272331 Free PMC article.
-
Mutational analysis of cysteine residues of the insect odorant co-receptor (Orco) from Drosophila melanogaster reveals differential effects on agonist- and odorant-tuning receptor-dependent activation.J Biol Chem. 2014 Nov 14;289(46):31837-31845. doi: 10.1074/jbc.M114.603993. Epub 2014 Sep 30. J Biol Chem. 2014. PMID: 25271160 Free PMC article.
-
Olfactory signaling in insects.Prog Mol Biol Transl Sci. 2015;130:37-54. doi: 10.1016/bs.pmbts.2014.11.002. Epub 2014 Dec 17. Prog Mol Biol Transl Sci. 2015. PMID: 25623336 Review.
-
The role of the coreceptor Orco in insect olfactory transduction.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Nov;199(11):897-909. doi: 10.1007/s00359-013-0837-3. Epub 2013 Jul 4. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23824225 Review.
Cited by
-
Odorant Inhibition in Mosquito Olfaction.iScience. 2019 Sep 27;19:25-38. doi: 10.1016/j.isci.2019.07.008. Epub 2019 Jul 12. iScience. 2019. PMID: 31349189 Free PMC article.
-
Human-like smelling of a rose scent using an olfactory receptor nanodisc-based bioelectronic nose.Sci Rep. 2018 Sep 17;8(1):13945. doi: 10.1038/s41598-018-32155-1. Sci Rep. 2018. PMID: 30224633 Free PMC article.
-
Natural volatiles preventing mosquito biting: An integrated screening platform for accelerated discovery of ORco antagonists.J Biol Chem. 2024 Dec;300(12):107939. doi: 10.1016/j.jbc.2024.107939. Epub 2024 Oct 29. J Biol Chem. 2024. PMID: 39476965 Free PMC article.
-
Odorant Receptors and Odorant-Binding Proteins as Insect Pest Control Targets: A Comparative Analysis.Front Physiol. 2018 Aug 24;9:1163. doi: 10.3389/fphys.2018.01163. eCollection 2018. Front Physiol. 2018. PMID: 30197600 Free PMC article. Review.
-
Odor coding of nestmate recognition in the eusocial ant Camponotus floridanus.J Exp Biol. 2020 Jan 28;223(Pt 2):jeb215400. doi: 10.1242/jeb.215400. J Exp Biol. 2020. PMID: 31900348 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

