Local Field Potentials: Myths and Misunderstandings
- PMID: 28018180
- PMCID: PMC5156830
- DOI: 10.3389/fncir.2016.00101
Local Field Potentials: Myths and Misunderstandings
Abstract
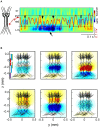
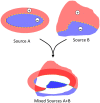
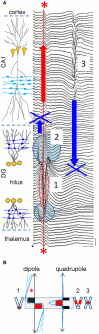
The intracerebral local field potential (LFP) is a measure of brain activity that reflects the highly dynamic flow of information across neural networks. This is a composite signal that receives contributions from multiple neural sources, yet interpreting its nature and significance may be hindered by several confounding factors and technical limitations. By and large, the main factor defining the amplitude of LFPs is the geometry of the current sources, over and above the degree of synchronization or the properties of the media. As such, similar levels of activity may result in potentials that differ in several orders of magnitude in different populations. The geometry of these sources has been experimentally inaccessible until intracerebral high density recordings enabled the co-activating sources to be revealed. Without this information, it has proven difficult to interpret a century's worth of recordings that used temporal cues alone, such as event or spike related potentials and frequency bands. Meanwhile, a collection of biophysically ill-founded concepts have been considered legitimate, which can now be corrected in the light of recent advances. The relationship of LFPs to their sources is often counterintuitive. For instance, most LFP activity is not local but remote, it may be larger further from rather than close to the source, the polarity does not define its excitatory or inhibitory nature, and the amplitude may increase when source's activity is reduced. As technological developments foster the use of LFPs, the time is now ripe to raise awareness of the need to take into account spatial aspects of these signals and of the errors derived from neglecting to do so.
Keywords: EEG; cell assembly; local field potentials; network oscillations; neuronal circuits; spatial discrimination; spontaneous activity; volume-conduction.
Figures



Similar articles
-
Spike-field Granger causality for hybrid neural data analysis.J Neurophysiol. 2019 Aug 1;122(2):809-822. doi: 10.1152/jn.00246.2019. Epub 2019 Jun 26. J Neurophysiol. 2019. PMID: 31242046
-
Modelling and analysis of local field potentials for studying the function of cortical circuits.Nat Rev Neurosci. 2013 Nov;14(11):770-85. doi: 10.1038/nrn3599. Nat Rev Neurosci. 2013. PMID: 24135696 Review.
-
Removal of spurious correlations between spikes and local field potentials.J Neurophysiol. 2011 Jan;105(1):474-86. doi: 10.1152/jn.00642.2010. Epub 2010 Nov 10. J Neurophysiol. 2011. PMID: 21068271
-
New uses of LFPs: Pathway-specific threads obtained through spatial discrimination.Neuroscience. 2015 Dec 3;310:486-503. doi: 10.1016/j.neuroscience.2015.09.054. Epub 2015 Sep 28. Neuroscience. 2015. PMID: 26415769 Review.
-
Model of low-pass filtering of local field potentials in brain tissue.Phys Rev E Stat Nonlin Soft Matter Phys. 2006 May;73(5 Pt 1):051911. doi: 10.1103/PhysRevE.73.051911. Epub 2006 May 19. Phys Rev E Stat Nonlin Soft Matter Phys. 2006. PMID: 16802971
Cited by
-
Distinct Roles of the Human Subthalamic Nucleus and Dorsal Pallidum in Parkinson's Disease Impulsivity.Biol Psychiatry. 2022 Feb 15;91(4):370-379. doi: 10.1016/j.biopsych.2021.03.002. Epub 2021 Mar 6. Biol Psychiatry. 2022. PMID: 33993998 Free PMC article.
-
Real-time suppression and amplification of frequency-specific neural activity using stimulation evoked oscillations.Brain Stimul. 2020 Nov-Dec;13(6):1732-1742. doi: 10.1016/j.brs.2020.09.017. Epub 2020 Oct 6. Brain Stimul. 2020. PMID: 33035727 Free PMC article.
-
Significance and Translational Value of High-Frequency Cortico-Basal Ganglia Oscillations in Parkinson's Disease.J Parkinsons Dis. 2019;9(1):183-196. doi: 10.3233/JPD-181480. J Parkinsons Dis. 2019. PMID: 30594935 Free PMC article.
-
Evolutionarily conserved anatomical and physiological properties of olfactory pathway through fourth-order neurons in a species of grasshopper (Hieroglyphus banian).J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019 Dec;205(6):813-838. doi: 10.1007/s00359-019-01369-7. Epub 2019 Sep 18. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019. PMID: 31535174
-
On the Comparison Between the Nc/CRN and the Ne/ERN.Front Hum Neurosci. 2022 Jun 23;15:788167. doi: 10.3389/fnhum.2021.788167. eCollection 2021. Front Hum Neurosci. 2022. PMID: 35812306 Free PMC article. Review.
References
-
- Benito N., Fernández-Ruiz A., Makarov V. A., Makarova J., Korovaichuk A., Herreras O. (2014). Spatial modules of coherent activity in pathway-specific LFPs in the hippocampus reflect topology and different modes of presynaptic synchronization. Cereb. Cortex 24, 1738–1752. 10.1093/cercor/bht022 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

