The Calcium-Sensing Receptor and the Parathyroid: Past, Present, Future
- PMID: 28018229
- PMCID: PMC5156698
- DOI: 10.3389/fphys.2016.00563
The Calcium-Sensing Receptor and the Parathyroid: Past, Present, Future
Abstract
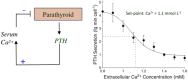
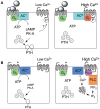
Parathyroid hormone (PTH) defends the extracellular fluid from hypocalcemia and has powerful and well-documented actions on the skeleton and renal tubular system. To achieve a satisfactory stable plasma calcium level, the secretion of PTH, and the resulting serum PTH level, is titrated carefully to the prevailing plasma ionized Ca2+ concentration via a Ca2+ sensing mechanism that mediates feedback inhibition of PTH secretion. Herein, I consider the properties of the parathyroid Ca2+ sensing mechanism, the identity of the Ca2+ sensor, the intracellular biochemical mechanisms that it controls, the manner of its integration with other components of the PTH secretion control mechanism, and its modulation by other nutrients. Together the well-established, recently elucidated, and yet-to-be discovered elements of the story constitute the past, present, and future of the parathyroid and its calcium-sensing receptor (CaSR).
Keywords: Calcimimetics; adenylate cyclase; calcilytics; calcium-sensing receptor; heterotrimeric G proteins; mineral metabolism; parathyroid; phospholipase C.
Figures
Similar articles
-
Autosomal Dominant Hypocalcemia (Hypoparathyroidism) Types 1 and 2.Front Physiol. 2016 Oct 18;7:458. doi: 10.3389/fphys.2016.00458. eCollection 2016. Front Physiol. 2016. PMID: 27803672 Free PMC article. Review.
-
[Calcimimetics].G Ital Nefrol. 2006 Jan-Feb;23(1):12-21. G Ital Nefrol. 2006. PMID: 16521071 Review. Italian.
-
Localization and function of the renal calcium-sensing receptor.Nat Rev Nephrol. 2016 Jul;12(7):414-25. doi: 10.1038/nrneph.2016.59. Epub 2016 May 9. Nat Rev Nephrol. 2016. PMID: 27157444 Review.
-
Calcilytic Ameliorates Abnormalities of Mutant Calcium-Sensing Receptor (CaSR) Knock-In Mice Mimicking Autosomal Dominant Hypocalcemia (ADH).J Bone Miner Res. 2015 Nov;30(11):1980-93. doi: 10.1002/jbmr.2551. Epub 2015 Jul 16. J Bone Miner Res. 2015. PMID: 25967373
-
Calcilytic NPSP795 Increases Plasma Calcium and PTH in an Autosomal Dominant Hypocalcemia Type 1 Mouse Model.JBMR Plus. 2020 Sep 7;4(10):e10402. doi: 10.1002/jbm4.10402. eCollection 2020 Oct. JBMR Plus. 2020. PMID: 33103030 Free PMC article.
Cited by
-
Heterogeneity of G protein activation by the calcium-sensing receptor.J Mol Endocrinol. 2021 Jun 21;67(2):41-53. doi: 10.1530/JME-21-0058. J Mol Endocrinol. 2021. PMID: 34077389 Free PMC article.
-
Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health.Int J Mol Sci. 2021 Mar 1;22(5):2478. doi: 10.3390/ijms22052478. Int J Mol Sci. 2021. PMID: 33804544 Free PMC article. Review.
-
Ex Vivo Intact Tissue Analysis Reveals Alternative Calcium-sensing Behaviors in Parathyroid Adenomas.J Clin Endocrinol Metab. 2021 Oct 21;106(11):3168-3183. doi: 10.1210/clinem/dgab524. J Clin Endocrinol Metab. 2021. PMID: 34272844 Free PMC article.
-
Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives.World J Gastroenterol. 2018 Sep 28;24(36):4119-4131. doi: 10.3748/wjg.v24.i36.4119. World J Gastroenterol. 2018. PMID: 30271078 Free PMC article. Review.
-
Nutritional and Pharmacological Targeting of the Calcium-Sensing Receptor Influences Chemically Induced Colitis in Mice.Nutrients. 2019 Dec 16;11(12):3072. doi: 10.3390/nu11123072. Nutrients. 2019. PMID: 31888253 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

