Inoculation of Soil with Plant Growth Promoting Bacteria Producing 1-Aminocyclopropane-1-Carboxylate Deaminase or Expression of the Corresponding acdS Gene in Transgenic Plants Increases Salinity Tolerance in Camelina sativa
- PMID: 28018305
- PMCID: PMC5159422
- DOI: 10.3389/fmicb.2016.01966
Inoculation of Soil with Plant Growth Promoting Bacteria Producing 1-Aminocyclopropane-1-Carboxylate Deaminase or Expression of the Corresponding acdS Gene in Transgenic Plants Increases Salinity Tolerance in Camelina sativa
Abstract
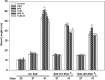
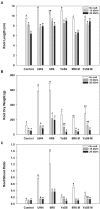
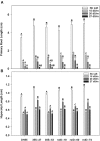
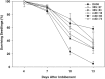
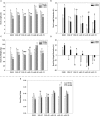
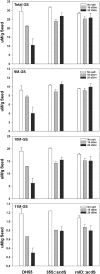
Camelina sativa (camelina) is an oilseed crop touted for use on marginal lands; however, it is no more tolerant of soil salinity than traditional crops, such as canola. Plant growth-promoting bacteria (PGPB) that produce 1-aminocyclopropane-1-carboxylate deaminase (ACC deaminase) facilitate plant growth in the presence of abiotic stresses by reducing stress ethylene. Rhizospheric and endophytic PGPB and the corresponding acdS- mutants of the latter were examined for their ability to enhance tolerance to salt in camelina. Stimulation of growth and tolerance to salt was correlated with ACC deaminase production. Inoculation of soil with wild-type PGPB led to increased shoot length in the absence of salt, and increased seed production by approximately 30-50% under moderately saline conditions. The effect of ACC deaminase was further examined in transgenic camelina expressing a bacterial gene encoding ACC deaminase (acdS) under the regulation of the CaMV 35S promoter or the root-specific rolD promoter. Lines expressing acdS, in particular those using the rolD promoter, showed less decline in root length and weight, increased seed production, better seed quality and higher levels of seed oil production under salt stress. This study clearly demonstrates the potential benefit of using either PGPB that produce ACC deaminase or transgenic plants expressing the acdS gene under the control of a root-specific promoter to facilitate plant growth, seed production and seed quality on land that is not normally suitable for the majority of crops due to high salt content.
Keywords: 1-aminocyclopropane-1-carboxylate deaminase; Camelina sativa; plant growth promoting bacteria; salinity tolerance; transgenic plants.
Figures







Similar articles
-
Gene Expression Patterns in Roots of Camelina sativa With Enhanced Salinity Tolerance Arising From Inoculation of Soil With Plant Growth Promoting Bacteria Producing 1-Aminocyclopropane-1-Carboxylate Deaminase or Expression the Corresponding acdS Gene.Front Microbiol. 2018 Jun 27;9:1297. doi: 10.3389/fmicb.2018.01297. eCollection 2018. Front Microbiol. 2018. PMID: 30013518 Free PMC article.
-
Gene expression patterns in shoots of Camelina sativa with enhanced salinity tolerance provided by plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene.Sci Rep. 2021 Feb 19;11(1):4260. doi: 10.1038/s41598-021-83629-8. Sci Rep. 2021. PMID: 33608579 Free PMC article.
-
1-Aminocyclopropane-1-carboxylate (ACC) Deaminase Gene in Pseudomonas azotoformans Is Associated with the Amelioration of Salinity Stress in Tomato.J Agric Food Chem. 2021 Jan 27;69(3):913-921. doi: 10.1021/acs.jafc.0c05628. Epub 2021 Jan 19. J Agric Food Chem. 2021. PMID: 33464897
-
The ACC deaminase-producing plant growth-promoting bacteria: Influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress.Physiol Plant. 2021 Dec;173(4):1992-2012. doi: 10.1111/ppl.13545. Epub 2021 Sep 17. Physiol Plant. 2021. PMID: 34487352 Review.
-
ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops.Microbiol Res. 2020 May;235:126439. doi: 10.1016/j.micres.2020.126439. Epub 2020 Feb 15. Microbiol Res. 2020. PMID: 32097862 Review.
Cited by
-
Realizing the Potential of Camelina sativa as a Bioenergy Crop for a Changing Global Climate.Plants (Basel). 2022 Mar 14;11(6):772. doi: 10.3390/plants11060772. Plants (Basel). 2022. PMID: 35336654 Free PMC article. Review.
-
CRISPR/Cas9 editing of three CRUCIFERIN C homoeologues alters the seed protein profile in Camelina sativa.BMC Plant Biol. 2019 Jul 4;19(1):292. doi: 10.1186/s12870-019-1873-0. BMC Plant Biol. 2019. PMID: 31272394 Free PMC article.
-
Effects of Klebsiella michiganensis LDS17 on Codonopsis pilosula growth, rhizosphere soil enzyme activities, and microflora, and genome-wide analysis of plant growth-promoting genes.Microbiol Spectr. 2024 May 2;12(5):e0405623. doi: 10.1128/spectrum.04056-23. Epub 2024 Apr 2. Microbiol Spectr. 2024. PMID: 38563743 Free PMC article.
-
Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress.Biology (Basel). 2022 Mar 12;11(3):437. doi: 10.3390/biology11030437. Biology (Basel). 2022. PMID: 35336811 Free PMC article. Review.
-
Cross-inoculation of rhizobiome from a congeneric ruderal plant imparts drought tolerance in maize (Zea mays) through changes in root morphology and proteome.Plant J. 2022 Jul;111(1):54-71. doi: 10.1111/tpj.15775. Epub 2022 Jun 8. Plant J. 2022. PMID: 35426964 Free PMC article.
References
-
- Abeles F. B., Morgan P. W., Saltveit M. E., Jr. (1992). Ethylene in Plant Biology (Second Edition). New York, NY: Academic Press.
-
- American Oil Chemists’ Society (2009). Determination of Saturated, cis-Monounsaturated, and cis-Polyunsaturated Fatty Acids in Marine and Other Oils Containing Long Chain Polyunsaturated Fatty Acids (PUFAs) by Capillary GLC. AOCS Official Method Ce li-07. Urbana, IL: AOCS.
LinkOut - more resources
Full Text Sources
Other Literature Sources

