Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells
- PMID: 28018313
- PMCID: PMC5156689
- DOI: 10.3389/fmicb.2016.01981
Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells
Abstract
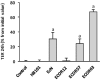
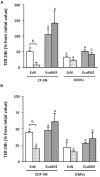
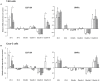
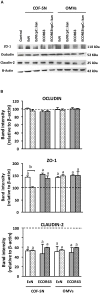
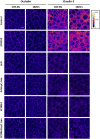
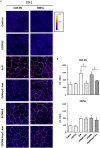
The gastrointestinal epithelial layer forms a physical and biochemical barrier that maintains the segregation between host and intestinal microbiota. The integrity of this barrier is critical in maintaining homeostasis in the body and its dysfunction is linked to a variety of illnesses, especially inflammatory bowel disease. Gut microbes, and particularly probiotic bacteria, modulate the barrier integrity by reducing gut permeability and reinforcing tight junctions. Probiotic Escherichia coli Nissle 1917 (EcN) is a good colonizer of the human gut with proven therapeutic efficacy in the remission of ulcerative colitis in humans. EcN positively modulates the intestinal epithelial barrier through upregulation and redistribution of the tight junction proteins ZO-1, ZO-2 and claudin-14. Upregulation of claudin-14 has been attributed to the secreted protein TcpC. Whether regulation of ZO-1 and ZO-2 is mediated by EcN secreted factors remains unknown. The aim of this study was to explore whether outer membrane vesicles (OMVs) released by EcN strengthen the epithelial barrier. This study includes other E. coli strains of human intestinal origin that contain the tcpC gene, such as ECOR63. Cell-free supernatants collected from the wild-type strains and from the derived tcpC mutants were fractionated into isolated OMVs and soluble secreted factors. The impact of these extracellular fractions on the epithelial barrier was evaluated by measuring transepithelial resistance and expression of several tight junction proteins in T-84 and Caco-2 polarized monolayers. Our results show that the strengthening activity of EcN and ECOR63 does not exclusively depend on TcpC. Both OMVs and soluble factors secreted by these strains promote upregulation of ZO-1 and claudin-14, and down-regulation of claudin-2. The OMVs-mediated effects are TcpC-independent. Soluble secreted TcpC contributes to the upregulation of ZO-1 and claudin-14, but this protein has no effect on the transcriptional regulation of claudin-2. Thus, in addition to OMVs and TcpC, other active factors released by these microbiota strains contribute to the reinforcement of the epithelial barrier.
Keywords: Escherichia coli; TcpC; gut microbes; intestinal barrier; membrane vesicles; phylogenetic group B2; probiotics; tight junctions.
Figures
References
-
- Arribas B., Rodríguez-Cabezas M. E., Camuesco D., Comalada M., Bailón E., Utrilla P., et al. (2009). A probiotic strain of Escherichia coli, Nissle 1917, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br. J. Pharmacol. 157 1024–1033. 10.1111/j.1476-5381.2009.00270.x - DOI - PMC - PubMed
-
- Cañas M. A., Giménez R., Fábrega M. J., Toloza L., Baldomà L., Badia J. (2016). Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS ONE 11:e0160374 10.1371/journal.pone.0160374 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

