The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation
- PMID: 28018338
- PMCID: PMC5149523
- DOI: 10.3389/fimmu.2016.00550
The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation
Abstract
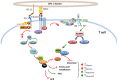
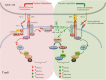
The immune system maintains a critically organized network to defend against foreign particles, while evading self-reactivity simultaneously. T lymphocytes function as effectors and play an important regulatory role to orchestrate the immune signals. Although central tolerance mechanism results in the removal of the most of the autoreactive T cells during thymic selection, a fraction of self-reactive lymphocytes escapes to the periphery and pose a threat to cause autoimmunity. The immune system evolved various mechanisms to constrain such autoreactive T cells and maintain peripheral tolerance, including T cell anergy, deletion, and suppression by regulatory T cells (TRegs). These effects are regulated by a complex network of stimulatory and inhibitory receptors expressed on T cells and their ligands, which deliver cell-to-cell signals that dictate the outcome of T cell encountering with cognate antigens. Among the inhibitory immune mediators, the pathway consisting of the programed cell death 1 (PD-1) receptor (CD279) and its ligands PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273) plays an important role in the induction and maintenance of peripheral tolerance and for the maintenance of the stability and the integrity of T cells. However, the PD-1:PD-L1/L2 pathway also mediates potent inhibitory signals to hinder the proliferation and function of T effector cells and have inimical effects on antiviral and antitumor immunity. Therapeutic targeting of this pathway has resulted in successful enhancement of T cell immunity against viral pathogens and tumors. Here, we will provide a brief overview on the properties of the components of the PD-1 pathway, the signaling events regulated by PD-1 engagement, and their consequences on the function of T effector cells.
Keywords: PD-1; PD-L1; T cell exhaustion; T cell responses; T cell tolerance; cancer immunology; cancer immunotherapy.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

