Detection of HLA Antibodies in Organ Transplant Recipients - Triumphs and Challenges of the Solid Phase Bead Assay
- PMID: 28018342
- PMCID: PMC5146910
- DOI: 10.3389/fimmu.2016.00570
Detection of HLA Antibodies in Organ Transplant Recipients - Triumphs and Challenges of the Solid Phase Bead Assay
Abstract
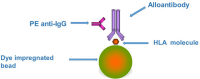
This review outlines the development of human leukocyte antigen (HLA) antibody detection assays and their use in organ transplantation in both antibody screening and crossmatching. The development of sensitive solid phase assays such as the enzyme-linked immunosorbent assay technique, and in particular the bead-based technology has revolutionized this field over the last 10-15 years. This revolution however has created a new paradigm in clinical decision making with respect to the detection of low level pretransplant HLA sensitization and its clinical relevance. The relative sensitivities of the assays used are discussed and the relevance of conflicting inter-assay results. Each assay has its advantages and disadvantages and these are discussed. Over the last decade, the bead-based assay utilizing the Luminex® fluorocytometer instrument has become established as the "gold standard" for HLA antibody testing. However, there are still unresolved issues surrounding this technique, such as the presence of denatured HLA molecules on the beads which reveal cryptic epitopes and the issue of appropriate fluorescence cut off values for positivity. The assay has been modified to detect complement binding (CB) in addition to non-complement binding (NCB) HLA antibodies although the clinical relevance of the CB and NCB IgG isotypes is not fully resolved. The increase sensitivity of the Luminex® bead assay over the complement-dependent cytotoxicity crossmatch has permitted the concept of the "virtual crossmatch" whereby the crossmatch is predicted to a high degree of accuracy based on the HLA antibody specificities detected by the solid phase assay. Dialog between clinicians and laboratory staff on an individual patient basis is essential for correct clinical decision making based on HLA antibody results obtained by the various techniques.
Keywords: CDC; ELISA; HLA antibody; Luminex; beads; transplantation.
Figures

Similar articles
-
Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation.Transplantation. 2013 Jan 15;95(1):19-47. doi: 10.1097/TP.0b013e31827a19cc. Transplantation. 2013. PMID: 23238534
-
Reappraisal of HLA antibody analysis and crossmatching in kidney transplantation.Clin Transpl. 2007:219-26. Clin Transpl. 2007. PMID: 18642453
-
Comparative analysis of Luminex-based donor-specific antibody mean fluorescence intensity values with complement-dependent cytotoxicity & flow crossmatch results in live donor renal transplantation.Indian J Med Res. 2017 Feb;145(2):222-228. doi: 10.4103/ijmr.IJMR_222_16. Indian J Med Res. 2017. PMID: 28639599 Free PMC article.
-
Review article: Luminex technology for HLA antibody detection in organ transplantation.Nephrology (Carlton). 2009 Apr;14(2):247-54. doi: 10.1111/j.1440-1797.2008.01074.x. Nephrology (Carlton). 2009. PMID: 19207861 Review.
-
Detection of alloantibody-mediated complement activation: A diagnostic advance in monitoring kidney transplant rejection?Clin Biochem. 2016 Mar;49(4-5):394-403. doi: 10.1016/j.clinbiochem.2015.05.024. Epub 2015 Jun 26. Clin Biochem. 2016. PMID: 26118475 Review.
Cited by
-
Pretransplant Kinetics of Anti-HLA Antibodies in Patients on the Waiting List for Kidney Transplantation.J Am Soc Nephrol. 2019 Nov;30(11):2262-2274. doi: 10.1681/ASN.2019060594. Epub 2019 Oct 25. J Am Soc Nephrol. 2019. PMID: 31653784 Free PMC article.
-
Risk Factors Influencing the Outcomes of Kidney Re-Transplantation.Ann Transplant. 2021 Jul 16;26:e928922. doi: 10.12659/AOT.928922. Ann Transplant. 2021. PMID: 34267171 Free PMC article.
-
Experimental Structures of Antibody/MHC-I Complexes Reveal Details of Epitopes Overlooked by Computational Prediction.J Immunol. 2024 Apr 15;212(8):1366-1380. doi: 10.4049/jimmunol.2300839. J Immunol. 2024. PMID: 38456672 Free PMC article.
-
Seventy-five years of service: an overview of the College of American Pathologists' proficiency testing program in histocompatibility and identity testing.Front Genet. 2023 Dec 19;14:1331169. doi: 10.3389/fgene.2023.1331169. eCollection 2023. Front Genet. 2023. PMID: 38169613 Free PMC article. Review.
-
Crossmatch assays in transplantation: Physical or virtual?: A review.Medicine (Baltimore). 2023 Dec 15;102(50):e36527. doi: 10.1097/MD.0000000000036527. Medicine (Baltimore). 2023. PMID: 38115324 Free PMC article. Review.
References
-
- Terasaki PI, Marchiori TH, Strazl TE. Serotyping of human lymphocyte antigens. Conference on Histocompatibility Testing. Washington, DC: Printing and Publishing Office National Academy of Sciences (1964). p. 83–95.
-
- Williams GM, Hume DM, Hudson RP, Jr, Morris PJ, Kano K, Milgorm F. Hyperacute renal-homograft rejection in man. N Engl J Med (1968) 279:611–8. - PubMed
-
- Fuller TC, Cosimi AB, Russell PS. Use of an antiglobulin-ATG reagent for detection of low levels of alloantibody. Improvement of allograft survival in presensitized recipients. Transplant Proc (1978) 10(2):463–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

