The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung
- PMID: 28018357
- PMCID: PMC5155013
- DOI: 10.3389/fimmu.2016.00591
The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung
Abstract
Radiation-induced pneumonitis and fibrosis are dose-limiting side effects of thoracic irradiation. Thoracic irradiation triggers acute and chronic environmental lung changes that are shaped by the damage response of resident cells, by the resulting reaction of the immune system, and by repair processes. Although considerable progress has been made during the last decade in defining involved effector cells and soluble mediators, the network of pathophysiological events and the cellular cross talk linking acute tissue damage to chronic inflammation and fibrosis still require further definition. Infiltration of cells from the innate and adaptive immune systems is a common response of normal tissues to ionizing radiation. Herein, lymphocytes represent a versatile and wide-ranged group of cells of the immune system that can react under specific conditions in various ways and participate in modulating the lung environment by adopting pro-inflammatory, anti-inflammatory, or even pro- or anti-fibrotic phenotypes. The present review provides an overview on published data about the role of lymphocytes in radiation-induced lung disease and related damage-associated pulmonary diseases with a focus on T lymphocytes and B lymphocytes. We also discuss the suspected dual role of specific lymphocyte subsets during the pneumonitic phase and fibrotic phase that is shaped by the environmental conditions as well as the interaction and the intercellular cross talk between cells from the innate and adaptive immune systems and (damaged) resident epithelial cells and stromal cells (e.g., endothelial cells, mesenchymal stem cells, and fibroblasts). Finally, we highlight potential therapeutic targets suited to counteract pathological lymphocyte responses to prevent or treat radiation-induced lung disease.
Keywords: fibrosis; lung; lymphocytes; pneumonitis; radiotherapy.
Figures
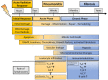
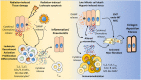
References
-
- Bartelink H, Rubens RD, van der Schueren E, Sylvester R. Hormonal therapy prolongs survival in irradiated locally advanced breast cancer: a European organization for research and treatment of cancer randomized phase III trial. J Clin Oncol (1997) 15(1):207–15. - PubMed
-
- Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European organization for research and treatment of cancer radiotherapy and gastrointestinal cooperative groups. J Clin Oncol (1997) 15(5):2040–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

