Endothelial Response to Glucocorticoids in Inflammatory Diseases
- PMID: 28018358
- PMCID: PMC5155119
- DOI: 10.3389/fimmu.2016.00592
Endothelial Response to Glucocorticoids in Inflammatory Diseases
Abstract
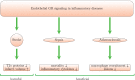
The endothelium plays a crucial role in inflammation. A balanced control of inflammation requires the action of glucocorticoids (GCs), steroidal hormones with potent cell-specific anti-inflammatory properties. Besides the classic anti-inflammatory effects of GCs on leukocytes, recent studies confirm that endothelial cells also represent an important target for GCs. GCs regulate different aspects of endothelial physiology including expression of adhesion molecules, production of pro-inflammatory cytokines and chemokines, and maintenance of endothelial barrier integrity. However, the regulation of endothelial GC sensitivity remains incompletely understood. In this review, we specifically examine the endothelial response to GCs in various inflammatory diseases ranging from multiple sclerosis, stroke, sepsis, and vasculitis to atherosclerosis. Shedding more light on the cross talk between GCs and endothelium will help to improve existing therapeutic strategies and develop new therapies better tailored to the needs of patients.
Keywords: adhesion molecules; cytokines; endothelium; glucocorticoid resistance; glucocorticoids; inflammation; tight junctions.
Figures

Similar articles
-
Dominance of the strongest: inflammatory cytokines versus glucocorticoids.Cytokine Growth Factor Rev. 2014 Feb;25(1):21-33. doi: 10.1016/j.cytogfr.2013.12.006. Epub 2013 Dec 24. Cytokine Growth Factor Rev. 2014. PMID: 24412262 Review.
-
Regulation of leukocyte-endothelial interactions by glucocorticoids.Ann N Y Acad Sci. 2002 Jun;966:108-18. doi: 10.1111/j.1749-6632.2002.tb04208.x. Ann N Y Acad Sci. 2002. PMID: 12114265 Review.
-
Effects of glucocorticoids on leukocytes: Genomic and non-genomic mechanisms.World J Clin Cases. 2022 Jul 26;10(21):7187-7194. doi: 10.12998/wjcc.v10.i21.7187. World J Clin Cases. 2022. PMID: 36158016 Free PMC article. Review.
-
Endothelial cells of the blood-brain barrier: a target for glucocorticoids and estrogens?Front Biosci. 2004 Jan 1;9:684-93. doi: 10.2741/1272. Front Biosci. 2004. PMID: 14766400 Review.
-
Glucocorticoids exert differential effects on the endothelium in an in vitro model of the blood-retinal barrier.Acta Ophthalmol. 2019 Mar;97(2):214-224. doi: 10.1111/aos.13909. Epub 2018 Aug 31. Acta Ophthalmol. 2019. PMID: 30168271
Cited by
-
Glucocorticoid-Like Activity of Escin: A New Mechanism for an Old Drug.Drug Des Devel Ther. 2021 Feb 24;15:699-704. doi: 10.2147/DDDT.S297501. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33658760 Free PMC article. Review.
-
In vivo vascular rarefaction and hypertension induced by dexamethasone are related to phosphatase PTP1B activation not endothelial metabolic changes.Free Radic Biol Med. 2020 May 20;152:689-696. doi: 10.1016/j.freeradbiomed.2020.01.012. Epub 2020 Jan 21. Free Radic Biol Med. 2020. PMID: 31978540 Free PMC article.
-
Plasmodium berghei NK65 in Combination with IFN-γ Induces Endothelial Glucocorticoid Resistance via Sustained Activation of p38 and JNK.Front Immunol. 2017 Sep 28;8:1199. doi: 10.3389/fimmu.2017.01199. eCollection 2017. Front Immunol. 2017. PMID: 29033931 Free PMC article.
-
SARS-CoV-2 Infection Is Associated with Reduced Krüppel-like Factor 2 in Human Lung Autopsy.Am J Respir Cell Mol Biol. 2021 Aug;65(2):222-226. doi: 10.1165/rcmb.2020-0564LE. Am J Respir Cell Mol Biol. 2021. PMID: 33971111 Free PMC article. No abstract available.
-
COVID-19 and thrombotic microangiopathies.Thromb Res. 2021 Jun;202:191-198. doi: 10.1016/j.thromres.2021.04.012. Epub 2021 Apr 20. Thromb Res. 2021. PMID: 33894421 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

