Narrow-Leafed Lupin (Lupinus angustifolius) β1- and β6-Conglutin Proteins Exhibit Antifungal Activity, Protecting Plants against Necrotrophic Pathogen Induced Damage from Sclerotinia sclerotiorum and Phytophthora nicotianae
- PMID: 28018392
- PMCID: PMC5161055
- DOI: 10.3389/fpls.2016.01856
Narrow-Leafed Lupin (Lupinus angustifolius) β1- and β6-Conglutin Proteins Exhibit Antifungal Activity, Protecting Plants against Necrotrophic Pathogen Induced Damage from Sclerotinia sclerotiorum and Phytophthora nicotianae
Abstract
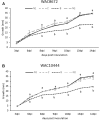
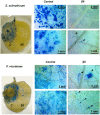
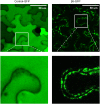
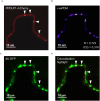
Vicilins (7S globulins) are seed storage proteins and constitute the main protein family in legume seeds, particularly in narrow-leafed lupin (Lupinus angustifolius L.; NLL), where seven vicilin genes, called β1- to β7-conglutin have been identified. Vicilins are involved in germination processes supplying amino acids for seedling growth and plant development, as well as in some cases roles in plant defense and protection against pathogens. The roles of NLL β-conglutins in plant defense are unknown. Here the potential role of five NLL β-conglutin family members in protection against necrotrophic fungal pathogens was investigated and it was demonstrated that recombinant purified 6xHis-tagged β1- and β6-conglutin proteins exhibited the strongest in vitro growth inhibitory activity against a range of necrotrophic fungal pathogens compared to β2, β3, and β4 conglutins. To examine activity in vivo, two representative necrotrophic pathogens, the fungus Sclerotinia sclerotiorum and oomycete Phytophthora nicotianae were used. Transient expression of β1- and β6-conglutin proteins in Nicotiana benthamiana leaves demonstrated in vivo growth suppression of both of these pathogens, resulting in low percentages of hyphal growth and elongation in comparison to control treated leaves. Cellular studies using β1- and β6-GFP fusion proteins showed these conglutins localized to the cell surface including plasmodesmata. Analysis of cellular death following S. sclerotiorum or P. nicotianae revealed both β1- and β6-conglutins suppressed pathogen induced cell death in planta and prevented pathogen induced suppression of the plant oxidative burst as determined by protein oxidation in infected compared to mock-inoculated leaves.
Keywords: 7S globulins; fungal pathogen; legume; oxidative stress; plant defense; seed storage protein; vicilins.
Figures


Similar articles
-
Identification and characterisation of seed storage protein transcripts from Lupinus angustifolius.BMC Plant Biol. 2011 Apr 4;11:59. doi: 10.1186/1471-2229-11-59. BMC Plant Biol. 2011. PMID: 21457583 Free PMC article.
-
Narrow-leafed lupin (Lupinus angustifolius L.) β-conglutin proteins modulate the insulin signaling pathway as potential type 2 diabetes treatment and inflammatory-related disease amelioration.Mol Nutr Food Res. 2017 May;61(5). doi: 10.1002/mnfr.201600819. Epub 2017 Feb 13. Mol Nutr Food Res. 2017. PMID: 28012244
-
Analysis of conglutin seed storage proteins across lupin species using transcriptomic, protein and comparative genomic approaches.BMC Plant Biol. 2015 Apr 19;15:106. doi: 10.1186/s12870-015-0485-6. BMC Plant Biol. 2015. PMID: 25902794 Free PMC article.
-
Nutritional composition and biological activity of narrow-leafed lupins (Lupinus angustifolius L.) hydrolysates and seeds.Food Chem. 2023 Sep 15;420:136104. doi: 10.1016/j.foodchem.2023.136104. Epub 2023 Apr 3. Food Chem. 2023. PMID: 37059020 Review.
-
Proteomics for exploiting diversity of lupin seed storage proteins and their use as nutraceuticals for health and welfare.J Proteomics. 2016 Jun 30;143:57-68. doi: 10.1016/j.jprot.2016.03.026. Epub 2016 Mar 18. J Proteomics. 2016. PMID: 26996462 Review.
Cited by
-
Irrigation affects characteristics of narrow-leaved lupin (Lupinus angustifolius L.) seeds.Planta. 2019 Jun;249(6):1731-1746. doi: 10.1007/s00425-019-03091-9. Epub 2019 Jan 25. Planta. 2019. PMID: 30684036 Free PMC article.
-
Genome-wide identification and expression analysis of the ALDH gene family and functional analysis of PaALDH17 in Prunus avium.Physiol Mol Biol Plants. 2024 Apr;30(4):633-645. doi: 10.1007/s12298-024-01444-7. Epub 2024 Apr 6. Physiol Mol Biol Plants. 2024. PMID: 38737320 Free PMC article.
-
Narrow-Leafed Lupin Main Allergen β-Conglutin (Lup an 1) Detection and Quantification Assessment in Natural and Processed Foods.Foods. 2019 Oct 18;8(10):513. doi: 10.3390/foods8100513. Foods. 2019. PMID: 31635336 Free PMC article.
-
Functional Association between Storage Protein Mobilization and Redox Signaling in Narrow-Leafed Lupin (Lupinus angustifolius L.) Seed Germination and Seedling Development.Genes (Basel). 2023 Sep 28;14(10):1889. doi: 10.3390/genes14101889. Genes (Basel). 2023. PMID: 37895238 Free PMC article.
-
Proteins in Relation to Vigor and Viability of White Lupin (Lupinus albus L.) Seed Stored for 26 Years.Front Plant Sci. 2017 Aug 11;8:1392. doi: 10.3389/fpls.2017.01392. eCollection 2017. Front Plant Sci. 2017. PMID: 28848591 Free PMC article.
References
-
- Agrios G. N. (2005). “Plant diseases caused by fungi,” in Plant Pathology 5th Edn ed. Agrios G. N. (San Diego, CA: Academic Press; ) 385–614.
-
- Alscher R. G., Donahue J. L., Cramer C. L. (1997). Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 100 224–233. 10.1111/j.1399-3054.1997.tb04778.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

