Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue
- PMID: 28018432
- PMCID: PMC5153503
- DOI: 10.1155/2016/4968724
Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue
Abstract
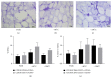
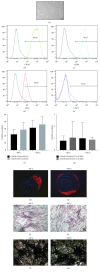
Osteoarthritis is characterized by loss of articular cartilage also due to reduced chondrogenic activity of mesenchymal stem cells (MSCs) from patients. Adipose tissue is an attractive source of MSCs (ATD-MSCs), representing an effective tool for reparative medicine, particularly for treatment of osteoarthritis, due to their chondrogenic and osteogenic differentiation capability. The treatment of symptomatic knee arthritis with ATD-MSCs proved effective with a single infusion, but multiple infusions could be also more efficacious. Here we studied some crucial aspects of adipose tissue banking procedures, evaluating ATD-MSCs viability, and differentiation capability after cryopreservation, to guarantee the quality of the tissue for multiple infusions. We reported that the presence of local anesthetic during lipoaspiration negatively affects cell viability of cryopreserved adipose tissue and cell growth of ATD-MSCs in culture. We observed that DMSO guarantees a faster growth of ATD-MSCs in culture than trehalose. At last, ATD-MSCs derived from fresh and cryopreserved samples at -80°C and -196°C showed viability and differentiation ability comparable to fresh samples. These data indicate that cryopreservation of adipose tissue at -80°C and -196°C is equivalent and preserves the content of ATD-MSCs in Stromal Vascular Fraction (SVF), guaranteeing the differentiation ability of ATD-MSCs.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures




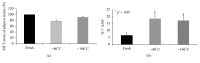
References
-
- Friedenstein A. J., Chailakhjan R. K., Lalykina K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics. 1970;3(4):393–403. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

