Long-term outcomes of stereotactic body radiation therapy (SBRT) with fiducial tracking for inoperable stage I non-small cell lung cancer (NSCLC)
- PMID: 28018523
- PMCID: PMC5149392
- DOI: 10.1007/s13566-016-0273-4
Long-term outcomes of stereotactic body radiation therapy (SBRT) with fiducial tracking for inoperable stage I non-small cell lung cancer (NSCLC)
Abstract
Background: Stereotactic body radiation therapy (SBRT) for stage I non-small cell lung cancer (NSCLC) is considered standard of care in the medically inoperable patient population. Multiple methods of SBRT delivery exist including fiducial-based tumor tracking, which allows for smaller treatment margins and avoidance of patient immobilization devices. We explore the long-term clinical outcomes of this novel fiducial-based SBRT method.
Methods: In this single institutional retrospective review, we detail the outcomes of medically inoperable pathologically confirmed stage I NSCLC. Patients were treated with the Cyberknife SBRT system using a planning target volume (PTV) defined as a 5-mm expansion from gross tumor volume (GTV) without creation of an internal target volume (ITV). Dose was delivered in three or five equal fractions of 10 to 20 Gy. Pretreatment and posttreatment pulmonary function test (PFT) changes and evidence of late radiological rib fractures were analyzed for the majority of patients. Actuarial local control, locoregional control, distant control, and overall survival were calculated using the Kaplan-Meier method.
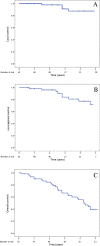
Results: Sixty-one patients with a median age of 75 years were available for analysis. The majority (80 %) of patients were deemed to be medically inoperable due to underlying pulmonary dysfunction. Eleven patients (18 %) developed symptomatic pneumothoraces secondary to fiducial placement under CT guidance, which precipitously dropped to 0 % following transition to bronchoscopic fiducial placement. The 2-year rib fracture risk was 21.4 % with a median time to rib fracture of 2.9 years. PFTs averaged over all patients and parameters demonstrated small absolute declines, 5.7 % averaged PFT decline, at approximately 1 year of follow-up, but only the diffusing capacity of lung for carbon monoxide (DLCO) demonstrated a statistically significant decline (10.29 vs. 9.01 mL/min/mmHg, p = 0.01). Five-year local control, locoregional control, and overall survival were 87.6, 71.8, and 39.3 %, respectively.
Conclusions: Despite reduced treatment margins and lack of patient immobilization, SBRT with fiducial-based tumor tracking achieves clinically comparable long-term outcomes to other linac-based SBRT approaches.
Keywords: Fiducial markers; Lung neoplasms; Non-small cell lung cancer; Pulmonary function test; Stereotactic body radiation therapy.
Conflict of interest statement
Compliance with ethical standards Funding source No funding support is associated with this study. Conflict of interests Brian T. Collins, MD, is a paid speaker for Accuray Inc. Jonathan W. Lischalk, MD; Stephanie M. Woo, BA; Shaan Kataria, MD; Nima Aghdam, MD; Ima Paydar, MD; Michael C. Repka, MD; and Eric D. Anderson, MD, have no competing interests. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The article does not contain any studies with human or animal subjects performed by any of the authors. The local Health Research Institutional Review Board (IRB) approved this retrospective analysis of an established departmental treatment approach. Malika Danner, MD contributed to the development and maintenance of the IRB documentation. Informed consent For this type of study, formal consent is not required.
Figures
References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. (2015) SEER Cancer Statistics Review, 1975-2012, National Cancer Institute
LinkOut - more resources
Full Text Sources
Other Literature Sources