Activation of Sphingosine 1-Phosphate Receptor 1 Enhances Hippocampus Neurogenesis in a Rat Model of Traumatic Brain Injury: An Involvement of MEK/Erk Signaling Pathway
- PMID: 28018679
- PMCID: PMC5153466
- DOI: 10.1155/2016/8072156
Activation of Sphingosine 1-Phosphate Receptor 1 Enhances Hippocampus Neurogenesis in a Rat Model of Traumatic Brain Injury: An Involvement of MEK/Erk Signaling Pathway
Abstract
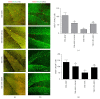
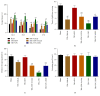
Among sphingosine 1-phosphate receptors (S1PRs) family, S1PR1 has been shown to be the most highly expressed subtype in neural stem cells (NSCs) and plays a crucial role in the migratory property of NSCs. Recent studies suggested that S1PR1 was expressed abundantly in the hippocampus, a specific neurogenic region in rodent brain for endogenous neurogenesis throughout life. However, the potential association between S1PR1 and neurogenesis in hippocampus following traumatic brain injury (TBI) remains unknown. In this study, the changes of hippocampal S1PR1 expression after TBI and their effects on neurogenesis and neurocognitive function were investigated, focusing on particularly the extracellular signal-regulated kinase (Erk) signaling pathway which had been found to regulate multiple properties of NSCs. The results showed that a marked upregulation of S1PR1 occurred with a peak at 7 days after trauma, revealing an enhancement of proliferation and neuronal differentiation of NSCs in hippocampus due to S1PR1 activation. More importantly, it was suggested that mitogen-activated protein kinase-Erk kinase (MEK)/Erk cascade was required for S1PR1-meidated neurogenesis and neurocognitive recovery following TBI. This study lays a preliminary foundation for future research on promoting hippocampal neurogenesis and improving TBI outcome.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures
Similar articles
-
MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway.Neurochem Res. 2019 Apr;44(4):811-828. doi: 10.1007/s11064-018-02714-z. Epub 2019 Jan 9. Neurochem Res. 2019. PMID: 30628018
-
Traumatic Brain Injury Stimulates Neural Stem Cell Proliferation via Mammalian Target of Rapamycin Signaling Pathway Activation.eNeuro. 2016 Nov 1;3(5):ENEURO.0162-16.2016. doi: 10.1523/ENEURO.0162-16.2016. eCollection 2016 Sep-Oct. eNeuro. 2016. PMID: 27822507 Free PMC article.
-
Curcumin alleviates neuroinflammation, enhances hippocampal neurogenesis, and improves spatial memory after traumatic brain injury.Brain Res Bull. 2020 Sep;162:84-93. doi: 10.1016/j.brainresbull.2020.05.009. Epub 2020 Jun 2. Brain Res Bull. 2020. PMID: 32502596
-
Insult-induced aberrant hippocampal neurogenesis: Functional consequences and possible therapeutic strategies.Behav Brain Res. 2019 Oct 17;372:112032. doi: 10.1016/j.bbr.2019.112032. Epub 2019 Jun 12. Behav Brain Res. 2019. PMID: 31199935 Review.
-
Strategies targeting endogenous neurogenic cell response to improve recovery following traumatic brain injury.Brain Res. 2016 Jun 1;1640(Pt A):104-113. doi: 10.1016/j.brainres.2016.01.055. Epub 2016 Feb 8. Brain Res. 2016. PMID: 26855258 Free PMC article. Review.
Cited by
-
MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway.Neurochem Res. 2019 Apr;44(4):811-828. doi: 10.1007/s11064-018-02714-z. Epub 2019 Jan 9. Neurochem Res. 2019. PMID: 30628018
-
Fingolimod reduces neuropathic pain behaviors in a mouse model of multiple sclerosis by a sphingosine-1 phosphate receptor 1-dependent inhibition of central sensitization in the dorsal horn.Pain. 2018 Feb;159(2):224-238. doi: 10.1097/j.pain.0000000000001106. Pain. 2018. PMID: 29140922 Free PMC article.
-
S1P/S1P Receptor Signaling in Neuromuscolar Disorders.Int J Mol Sci. 2019 Dec 17;20(24):6364. doi: 10.3390/ijms20246364. Int J Mol Sci. 2019. PMID: 31861214 Free PMC article. Review.
-
The GPCR adaptor protein Norbin regulates S1PR1 trafficking and the morphology, cell cycle and survival of PC12 cells.Sci Rep. 2023 Oct 25;13(1):18237. doi: 10.1038/s41598-023-45148-6. Sci Rep. 2023. PMID: 37880240 Free PMC article.
-
Microglia-induced neuroinflammation in hippocampal neurogenesis following traumatic brain injury.Heliyon. 2024 Aug 8;10(16):e35869. doi: 10.1016/j.heliyon.2024.e35869. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220913 Free PMC article. Review.
References
-
- Yamaguchi M., Seki T., Imayoshi I., et al. Neural stem cells and neuro/gliogenesis in the central nervous system: understanding the structural and functional plasticity of the developing, mature, and diseased brain. Journal of Physiological Sciences. 2016;66(3):179–206. doi: 10.1007/s12576-015-0421-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

