Noninvasive Submental Fat Compartment Treatment
- PMID: 28018773
- PMCID: PMC5172481
- DOI: 10.1097/GOX.0000000000001155
Noninvasive Submental Fat Compartment Treatment
Abstract
Background: KYBELLA, ATX-101, is an injectable form of sodium deoxycholic acid. It is currently the only Food and Drug Administration-approved injectable drug for the reduction of submental fat.
Objectives: A literature review and discussion of the treatment of submental fat.
Results: KYBELLA is a well-tolerated alternative for the treatment of submental fat.
Conclusions: KYBELLA is a safe and efficacious, first in class, injectable drug for the reduction of submental fat.
Conflict of interest statement
The author has no financial interest to declare in relation to the content of this article. The Article Processing Charge for this proceeding was paid for by Allergan plc, as part of an unrestricted educational grant to support the entire Cosmetic Boot Camp 2016 Supplement. Allergan plc had no involvement in the production, selection, or review of this proceeding supplement.
Figures


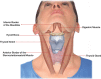


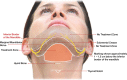
References
-
- Jones DH, Carruthers J, Joseph JH, et al. REFINE-1, a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial with ATX-101, an injectable drug for submental fat reduction. Dermatol Surg. 2016;42:38–49. - PubMed
-
- Duncan D, Rotunda AM. Injectable therapies for localized fat loss: state of the art, clinics in plastic surgery. Clin Plast Surg. 2011;38:489–501. - PubMed
-
- Beer K, Donofrio L, Gross TM, et al. Clinically meaningful reduction in submental fat during and after treatment with ATX-101 in the US/CAN phase 3 trials, REFINE-1, and REFINE-2. Presented at 73rd Annual Meeting of the American Academy of Dermatology; March 20–24, 2015; San Francisco, CA.
-
- Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4–S9. - PubMed
-
- Ascher B, Hoffmann K, Walker P, et al. Efficacy, patient-reported outcomes and safety profile of ATX-101 (deoxycholic acid), an injectable drug for the reduction of unwanted submental fat: results from a phase III, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2014;28:1707–1715. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
