Technical Considerations for Filler and Neuromodulator Refinements
- PMID: 28018778
- PMCID: PMC5172486
- DOI: 10.1097/GOX.0000000000001178
Technical Considerations for Filler and Neuromodulator Refinements
Abstract
Background: The toolbox for cosmetic practitioners is growing at an unprecedented rate. There are novel products every year and expanding off-label indications for neurotoxin and soft-tissue filler applications. Consequently, aesthetic physicians are increasingly challenged by the task of selecting the most appropriate products and techniques to achieve optimal patient outcomes. Methods: We employed a PubMed literature search of facial injectables from the past 10 years (2005-2015), with emphasis on those articles embracing evidence-based medicine. We evaluated the scientific background of every product and the physicochemical properties that make each one ideal for specific indications. The 2 senior authors provide commentary regarding their clinical experience with specific technical refinements of neuromodulators and soft-tissue fillers. Results: Neurotoxins and fillers are characterized by unique physical characteristics that distinguish each product. This results in subtle but important differences in their clinical applications. Specific indications and recommendations for the use of the various neurotoxins and soft-tissue fillers are reviewed. The discussion highlights refinements in combination treatments and product physical modifications, according to specific treatment zones. Conclusions: The field of facial aesthetics has evolved dramatically, mostly secondary to our increased understanding of 3-dimensional structural volume restoration. Our work reviews Food and Drug Administration-approved injectables. In addition, we describe how to modify products to fulfill specific indications such as treatment of the mid face, décolletage, hands, and periorbital regions. Although we cannot directly evaluate the duration or exact physical properties of blended products, we argue that "product customization" is safe and provides natural results with excellent patient outcomes.
Conflict of interest statement
Disclosure: José Raúl Montes has served as a consultant, speaker, and trainer for Allergan, Galderma, Merz, and Valeant. Ivona Percec has served as a consultant for Galderma. Neither of the other authors has any financial disclosures. The Article Processing Charge for this proceeding was paid for by Allergan plc, as part of an unrestricted educational grant to support the entire Cosmetic Boot Camp 2016 Supplement. Allergan plc had no involvement in the production, selection, or review of this proceeding supplement.
Figures






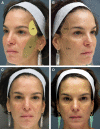
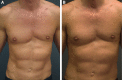
References
-
- 2014 Plastic Surgery Statistics Report. American Society of Plastic Surgeons. Available at: https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2014/plastic-surge.... Accessed January 8, 2016.
-
- Gart MS, Gutowski KA. Aesthetic uses of neuromodulators: current uses and future directions. Plast Reconstr Surg. 2015;136:62S–71S. - PubMed
-
- BOTOX® Cosmetic Prescribing Information. Allergan. Available at: http://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf. Accessed January 19, 2016.
-
- Full Prescribing Information – Dysport. Galderma. Available at: http://www.galderma.com.au/Portals/4/PIs%20and%20CMIs/April%2016%20uploa.... Accessed January 19, 2016.
-
- Prescribing Information – Xeomin. Merz Pharmaceuticals. Available at: http://www.xeominaesthetic.com/wp-content/uploads/XEOMIN-Prescribing-Inf....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
