Mycobacterium bovis Induces Endoplasmic Reticulum Stress Mediated-Apoptosis by Activating IRF3 in a Murine Macrophage Cell Line
- PMID: 28018864
- PMCID: PMC5149527
- DOI: 10.3389/fcimb.2016.00182
Mycobacterium bovis Induces Endoplasmic Reticulum Stress Mediated-Apoptosis by Activating IRF3 in a Murine Macrophage Cell Line
Abstract
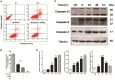
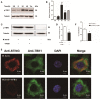
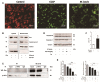
Mycobacterium bovis (M. bovis) is highly adapted to macrophages and has developed multiple mechanisms to resist intracellular assaults. However, the host cells in turn deploy a multipronged defense mechanism to control bacterial infection. Endoplasmic reticulum (ER) stress-mediated apoptosis is one such primary defense mechanism. However, the role of interferon regulatory factor 3 (IRF3) between ER stress and apoptosis during M. bovis infection is unknown. Here, we demonstrate that M. bovis effectively induced apoptosis in murine macrophages. Caspase-12, caspase-9, and caspase-3 were activated over a 48 h infection period. The splicing of XBP-1 mRNA and the level of phosphorylation of eIF2α, indicators of ER stress, significantly increased at early time points after M. bovis infection. The expansion of the ER compartment, a morphological hallmark of ER stress, was observed at 6 h. Pre-treatment of Raw 264.7 cells with 4-PBA (an ER stress-inhibitor) reduced the activation of the ER stress indicators, caspase activation and its downstream poly (ADP-ribose) polymerase (PARP) cleavage, phosphorylation of TBK1 and IRF3 and cytoplasmic co-localization of STING and TBK1. M. bovis infection led to the interaction of activated IRF3 and cytoplasmic Bax leading to mitochondrial damage. Role of IRF3 in apoptosis was further confirmed by blocking this molecule with BX-795 that showed significant reduction expression of caspase-8 and caspase-3. Intracellular survival of M. bovis increased in response to 4-PBA and BX-795. These findings indicate that STING-TBK1-IRF3 pathway mediates a crosstalk between ER stress and apoptosis during M. bovis infection, which can effectively control intracellular bacteria.
Keywords: ER stress; IRF3; M. bovis; apoptosis; mycobacterium.
Figures




Similar articles
-
BAG2 ameliorates endoplasmic reticulum stress-induced cell apoptosis in Mycobacterium tuberculosis-infected macrophages through selective autophagy.Autophagy. 2020 Aug;16(8):1453-1467. doi: 10.1080/15548627.2019.1687214. Epub 2019 Nov 11. Autophagy. 2020. PMID: 31711362 Free PMC article.
-
Reactive oxygen species-mediated endoplasmic reticulum stress response induces apoptosis of Mycobacterium avium-infected macrophages by activating regulated IRE1-dependent decay pathway.Cell Microbiol. 2019 Dec;21(12):e13094. doi: 10.1111/cmi.13094. Epub 2019 Aug 16. Cell Microbiol. 2019. PMID: 31386788 Free PMC article.
-
The PGRS Domain of Mycobacterium tuberculosis PE_PGRS Protein Rv0297 Is Involved in Endoplasmic Reticulum Stress-Mediated Apoptosis through Toll-Like Receptor 4.mBio. 2018 Jun 19;9(3):e01017-18. doi: 10.1128/mBio.01017-18. mBio. 2018. PMID: 29921671 Free PMC article.
-
STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease.Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16544-9. doi: 10.1073/pnas.1308331110. Epub 2013 Sep 19. Proc Natl Acad Sci U S A. 2013. PMID: 24052526 Free PMC article.
-
Crosstalk of the Caspase Family and Mammalian Target of Rapamycin Signaling.Int J Mol Sci. 2021 Jan 15;22(2):817. doi: 10.3390/ijms22020817. Int J Mol Sci. 2021. PMID: 33467535 Free PMC article. Review.
Cited by
-
Multiple unfolded protein response pathways cooperate to link cytosolic dsDNA release to stimulator of interferon gene activation.Front Immunol. 2024 Jul 19;15:1358462. doi: 10.3389/fimmu.2024.1358462. eCollection 2024. Front Immunol. 2024. PMID: 39100663 Free PMC article.
-
Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage.Reprod Biol Endocrinol. 2018 Sep 3;16(1):85. doi: 10.1186/s12958-018-0404-4. Reprod Biol Endocrinol. 2018. PMID: 30176887 Free PMC article.
-
The Advancing of Selenium Nanoparticles Against Infectious Diseases.Front Pharmacol. 2021 Jul 30;12:682284. doi: 10.3389/fphar.2021.682284. eCollection 2021. Front Pharmacol. 2021. PMID: 34393776 Free PMC article. Review.
-
How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis.Int J Mol Sci. 2023 Feb 3;24(3):3029. doi: 10.3390/ijms24033029. Int J Mol Sci. 2023. PMID: 36769349 Free PMC article. Review.
-
Impact of STING Inflammatory Signaling during Intracellular Bacterial Infections.Cells. 2021 Dec 28;11(1):74. doi: 10.3390/cells11010074. Cells. 2021. PMID: 35011636 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

