The Mutational Landscape of the Oncogenic MZF1 SCAN Domain in Cancer
- PMID: 28018905
- PMCID: PMC5156680
- DOI: 10.3389/fmolb.2016.00078
The Mutational Landscape of the Oncogenic MZF1 SCAN Domain in Cancer
Abstract
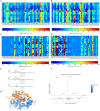
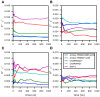
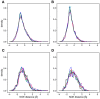
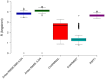
SCAN domains in zinc-finger transcription factors are crucial mediators of protein-protein interactions. Up to 240 SCAN-domain encoding genes have been identified throughout the human genome. These include cancer-related genes, such as the myeloid zinc finger 1 (MZF1), an oncogenic transcription factor involved in the progression of many solid cancers. The mechanisms by which SCAN homo- and heterodimers assemble and how they alter the transcriptional activity of zinc-finger transcription factors in cancer and other diseases remain to be investigated. Here, we provide the first description of the conformational ensemble of the MZF1 SCAN domain cross-validated against NMR experimental data, which are probes of structure and dynamics on different timescales. We investigated the protein-protein interaction network of MZF1 and how it is perturbed in different cancer types by the analyses of high-throughput proteomics and RNASeq data. Collectively, we integrated many computational approaches, ranging from simple empirical energy functions to all-atom microsecond molecular dynamics simulations and network analyses to unravel the effects of cancer-related substitutions in relation to MZF1 structure and interactions.
Keywords: FoldX; RNAseq; TCGA; cancer mutations; molecular dynamics; protein structure network; saturation mutagenesis; transcription factors.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

