Prefrontal hemodynamic mapping by functional near-infrared spectroscopy in response to thermal stimulations over three body sites
- PMID: 28018934
- PMCID: PMC5166717
- DOI: 10.1117/1.NPh.3.4.045008
Prefrontal hemodynamic mapping by functional near-infrared spectroscopy in response to thermal stimulations over three body sites
Abstract
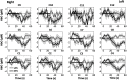
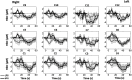
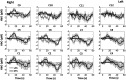
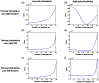
Functional near-infrared spectroscopy (fNIRS) was used to examine hemodynamic responses in the prefrontal cortex (PFC) during noxious thermal pain, induced by thermal stimulations over three different body sites over the right forearm, right temporomandibular joint, and left forearm. Functional NIRS measurements were obtained from three groups of healthy volunteers, one group for each body region. Each group was subjected to both low-pain stimulation (LPS) and high-pain stimulation (HPS) by a [Formula: see text] thermode of a temperature-controlled thermal stimulator over the respective three body sites. Our results showed that HPS given at three sites induced significant increases ([Formula: see text]) in oxy-hemoglobin concentration ([Formula: see text]) in the PFC with similar temporal patterns in relatively spread PFC areas. In contrast, LPS did not cause any significant [Formula: see text] in the PFC of any subject group. Our observed PFC activations induced by acute HPS were generally consistent with previous reports by fMRI studies. The study also found a peculiar global trend of postpain deactivation in the PFC, which is attributed to global vasoconstriction due to acute nocuous pain. Overall, these results indicate that hemodynamic activities in PFC exhibit consistent temporal and spatial patterns in response to acute thermal stimulation given across all three body sites.
Keywords: functional near-infrared spectroscopy; innocuous pain; noxious pain; prefrontal hemodynamic mapping; thermal stimulations.
Figures

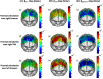

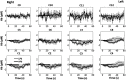

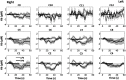
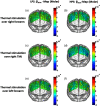
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

