Hypoxia sensing through β-adrenergic receptors
- PMID: 28018974
- PMCID: PMC5161214
- DOI: 10.1172/jci.insight.90240
Hypoxia sensing through β-adrenergic receptors
Abstract
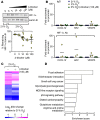
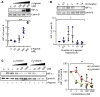
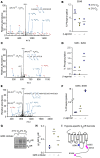
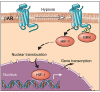
Life-sustaining responses to low oxygen, or hypoxia, depend on signal transduction by HIFs, but the underlying mechanisms by which cells sense hypoxia are not completely understood. Based on prior studies suggesting a link between the β-adrenergic receptor (β-AR) and hypoxia responses, we hypothesized that the β-AR mediates hypoxia sensing and is necessary for HIF-1α accumulation. Beta blocker treatment of mice suppressed hypoxia induction of renal HIF-1α accumulation, erythropoietin production, and erythropoiesis in vivo. Likewise, beta blocker treatment of primary human endothelial cells in vitro decreased hypoxia-mediated HIF-1α accumulation and binding to target genes and the downstream hypoxia-inducible gene expression. In mechanistic studies, cAMP-activated PKA and/or GPCR kinases (GRK), which both participate in β-AR signal transduction, were investigated. Direct activation of cAMP/PKA pathways did not induce HIF-1α accumulation, and inhibition of PKA did not blunt HIF-1α induction by hypoxia. In contrast, pharmacological inhibition of GRK, or expression of a GRK phosphorylation-deficient β-AR mutant in cells, blocked hypoxia-mediated HIF-1α accumulation. Mass spectrometry-based quantitative analyses revealed a hypoxia-mediated β-AR phosphorylation barcode that was different from the classical agonist phosphorylation barcode. These findings indicate that the β-AR is fundamental to the molecular and physiological responses to hypoxia.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

