Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors
- PMID: 28019091
- PMCID: PMC5270302
- DOI: 10.1002/psp4.12143
Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors
Abstract
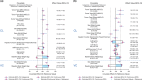
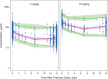
Nivolumab is a fully human monoclonal antibody that inhibits programmed death-1 activation. The clinical pharmacology profile of nivolumab was analyzed by a population pharmacokinetics model that assessed covariate effects on nivolumab concentrations in 1,895 patients who received 0.3-10.0 mg/kg nivolumab in 11 clinical trials. Nivolumab pharmacokinetics is linear with a time-varying clearance. A full covariate model was developed to assess covariate effects on pharmacokinetic parameters. Nivolumab clearance and volume of distribution increase with body weight. The final model included the effects of baseline performance status (PS), baseline body weight, and baseline estimated glomerular filtration rate (eGFR), sex, and race on clearance, and effects of baseline body weight and sex on volume of distribution in the central compartment. Sex, PS, baseline eGFR, age, race, baseline lactate dehydrogenase, mild hepatic impairment, tumor type, tumor burden, and programmed death ligand-1 expression had a significant but not clinically relevant (<20%) effect on nivolumab clearance.
© 2016 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

References
-
- Sharpe, A.H. , Wherry, E.J. , Ahmed, R. & Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 8, 239–245 (2007). - PubMed
-
- Ott, P.A. , Hodi, F.S. & Robert, C. CTLA‐4 and PD‐1/PD‐L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 19, 5300–5309 (2013). - PubMed
-
- Jurado, J.O. et al Programmed death (PD)−1:PD‐ligand 1/PD‐ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 181, 116–125 (2008). - PubMed
-
- Wang, C. et al In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res. 2, 846–856 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

