Designer vaccine nanodiscs for personalized cancer immunotherapy
- PMID: 28024156
- PMCID: PMC5374005
- DOI: 10.1038/nmat4822
Designer vaccine nanodiscs for personalized cancer immunotherapy
Abstract
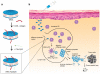
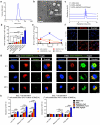
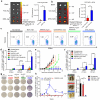
Despite the tremendous potential of peptide-based cancer vaccines, their efficacy has been limited in humans. Recent innovations in tumour exome sequencing have signalled the new era of personalized immunotherapy with patient-specific neoantigens, but a general methodology for stimulating strong CD8α+ cytotoxic T-lymphocyte (CTL) responses remains lacking. Here we demonstrate that high-density lipoprotein-mimicking nanodiscs coupled with antigen (Ag) peptides and adjuvants can markedly improve Ag/adjuvant co-delivery to lymphoid organs and sustain Ag presentation on dendritic cells. Strikingly, nanodiscs elicited up to 47-fold greater frequencies of neoantigen-specific CTLs than soluble vaccines and even 31-fold greater than perhaps the strongest adjuvant in clinical trials (that is, CpG in Montanide). Moreover, multi-epitope vaccination generated broad-spectrum T-cell responses that potently inhibited tumour growth. Nanodiscs eliminated established MC-38 and B16F10 tumours when combined with anti-PD-1 and anti-CTLA-4 therapy. These findings represent a new powerful approach for cancer immunotherapy and suggest a general strategy for personalized nanomedicine.
Figures


References
-
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. - PubMed
-
- Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

