Developmental broadening of inhibitory sensory maps
- PMID: 28024159
- PMCID: PMC5510602
- DOI: 10.1038/nn.4467
Developmental broadening of inhibitory sensory maps
Abstract
Sensory maps are created by networks of neuronal responses that vary with their anatomical position, such that representations of the external world are systematically and topographically organized in the brain. Current understanding from studying excitatory maps is that maps are sculpted and refined throughout development and/or through sensory experience. Investigating the mouse olfactory bulb, where ongoing neurogenesis continually supplies new inhibitory granule cells into existing circuitry, we isolated the development of sensory maps formed by inhibitory networks. Using in vivo calcium imaging of odor responses, we compared functional responses of both maturing and established granule cells. We found that, in contrast to the refinement observed for excitatory maps, inhibitory sensory maps became broader with maturation. However, like excitatory maps, inhibitory sensory maps are sensitive to experience. These data describe the development of an inhibitory sensory map as a network, highlighting the differences from previously described excitatory maps.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


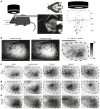

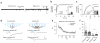
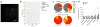


Comment in
-
The maps they are a-changin': plasticity in odor representation in interneurons.Nat Neurosci. 2017 Jan 27;20(2):128-129. doi: 10.1038/nn.4484. Nat Neurosci. 2017. PMID: 28127039 No abstract available.
References
-
- Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. - PubMed
-
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. - PubMed
-
- Iwasato T, et al. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

