qNMR for profiling the production of fungal secondary metabolites
- PMID: 28024162
- PMCID: PMC5459663
- DOI: 10.1002/mrc.4571
qNMR for profiling the production of fungal secondary metabolites
Abstract
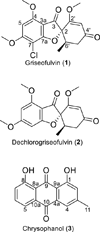
Analysis of complex mixtures is a common challenge in natural products research. Quantitative nuclear magnetic resonance spectroscopy offers analysis of complex mixtures at early stages and with benefits that are orthogonal to more common methods of quantitation, including ultraviolet absorption spectroscopy and mass spectrometry. Several experiments were conducted to construct a methodology for use in analysis of extracts of fungal cultures. A broadly applicable method was sought for analysis of both pure and complex samples through use of an externally calibrated method. This method has the benefit of not contaminating valuable samples with the calibrant, and it passed scrutiny for line fitting and reproducibility. The method was implemented to measure the yield of griseofulvin and dechlorogriseofulvin from three fungal isolates. An isolate of Xylaria cubensis (coded MSX48662) was found to biosynthesize griseofulvin in the greatest yield, 149 ± 8 mg per fermentation, and was selected for further supply experiments. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: 1H NMR; NMR; fungi; griseofulvin; qNMR; secondary metabolites.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures
Similar articles
-
Universal quantitative NMR analysis of complex natural samples.Curr Opin Biotechnol. 2014 Feb;25:51-9. doi: 10.1016/j.copbio.2013.08.004. Epub 2013 Sep 14. Curr Opin Biotechnol. 2014. PMID: 24484881 Free PMC article. Review.
-
Metabolomics and dereplication strategies in natural products.Methods Mol Biol. 2013;1055:227-44. doi: 10.1007/978-1-62703-577-4_17. Methods Mol Biol. 2013. PMID: 23963915
-
OSMAC Strategy Integrated with Molecular Networking for Accessing Griseofulvin Derivatives from Endophytic Fungi of Moquiniastrum polymorphum (Asteraceae).Molecules. 2021 Dec 2;26(23):7316. doi: 10.3390/molecules26237316. Molecules. 2021. PMID: 34885898 Free PMC article.
-
Spatial and Temporal Profiling of Griseofulvin Production in Xylaria cubensis Using Mass Spectrometry Mapping.Front Microbiol. 2016 Apr 26;7:544. doi: 10.3389/fmicb.2016.00544. eCollection 2016. Front Microbiol. 2016. PMID: 27199902 Free PMC article.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy For Metabolic Profiling of Medicinal Plants and Their Products.Crit Rev Anal Chem. 2016 Sep 2;46(5):400-12. doi: 10.1080/10408347.2015.1106932. Epub 2015 Nov 17. Crit Rev Anal Chem. 2016. PMID: 26575437 Review.
Cited by
-
Isolation and Determination of Fomentariol: Novel Potential Antidiabetic Drug from Fungal Material.J Anal Methods Chem. 2018 Feb 20;2018:2434691. doi: 10.1155/2018/2434691. eCollection 2018. J Anal Methods Chem. 2018. PMID: 29675285 Free PMC article.
-
Chemoselective fluorination and chemoinformatic analysis of griseofulvin: Natural vs fluorinated fungal metabolites.Bioorg Med Chem. 2017 Oct 15;25(20):5238-5246. doi: 10.1016/j.bmc.2017.07.041. Epub 2017 Jul 28. Bioorg Med Chem. 2017. PMID: 28802670 Free PMC article.
-
Fungal Guttation, a Source of Bioactive Compounds, and Its Ecological Role-A Review.Biomolecules. 2021 Aug 25;11(9):1270. doi: 10.3390/biom11091270. Biomolecules. 2021. PMID: 34572483 Free PMC article. Review.
References
-
- Frisvad JC. In: Fungal Secondary Metabolism: Methods and Protocols. Keller NP, Turner G, editors. New York: Springer; 2012. pp. 47–77.
-
- Xu F, Tao W, Cheng L, Guo L. Biochem. Eng. J. 2006;31:67–73.
-
- Cai M-H, Zhou X-S, Sun X-Q, Tao K-J, Zhang Y-X. J. Ind. Microbiol. Biotechnol. 2009;36:381–389. - PubMed
-
- Revankar MS, Lele SS. Process. Biochem. 2006;41:581–588.
-
- Deswal D, Khasa YP, Kuhad RC. Bioresour. Technol. 2011;102:6065–6072. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

