Impact of azithromycin on the clinical and antimicrobial effectiveness of tobramycin in the treatment of cystic fibrosis
- PMID: 28025037
- PMCID: PMC5492972
- DOI: 10.1016/j.jcf.2016.12.003
Impact of azithromycin on the clinical and antimicrobial effectiveness of tobramycin in the treatment of cystic fibrosis
Abstract
Background: Concomitant use of oral azithromycin and inhaled tobramycin occurs in approximately half of US cystic fibrosis (CF) patients. Recent data suggest that this combination may be antagonistic.
Methods: Test the hypothesis that azithromycin reduces the clinical benefits of tobramycin by analyses of clinical trial data, in vitro modeling of P. aeruginosa antibiotic killing, and regulation of the MexXY efflux pump.
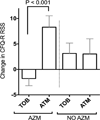
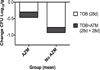
Results: Ongoing administration of azithromycin associates with reduced ability of inhaled tobramycin, as compared with aztreonam, to improve lung function and quality of life in a completed clinical trial. In users of azithromycin FEV1 (L) increased 0.8% during a 4-week period of inhaled tobramycin and an additional 6.4% during a subsequent 4-week period of inhaled aztreonam (P<0.005). CFQ-R respiratory symptom score decreased 1.8 points during inhaled tobramycin and increased 8.3 points during subsequent inhaled aztreonam (P<0.001). A smaller number of trial participants not using azithromycin had similar improvement in lung function and quality of life scores during inhaled tobramycin and inhaled aztreonam. In vitro, azithromycin selectively reduced the bactericidal effects tobramycin in cultures of clinical strains of P. aeruginosa, while up regulating antibiotic resistance through MexXY efflux.
Conclusions: Azithromycin appears capable of reducing the antimicrobial benefits of tobramycin by inducing adaptive bacterial stress responses in P. aeruginosa, suggesting that these medications together may not be optimal chronic therapy for at least some patients.
Keywords: Azithromycin; Clinical trial; Cystic fibrosis; Drug interaction; Inhaled antibiotics; MexXY; Pseudomonas aeruginosa; Tobramycin.
Copyright © 2016 European Cystic Fibrosis Society. Published by Elsevier B.V. All rights reserved.
Figures

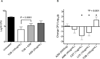




References
-
- Mogayzel PJ, Jr, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–689. - PubMed
-
- Ramsey BWPM, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. - PubMed
-
- Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

