Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial
- PMID: 28025189
- PMCID: PMC5381596
- DOI: 10.1093/eurheartj/ehw543
Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial
Abstract
Aims: Cardiopoietic cells, produced through cardiogenic conditioning of patients' mesenchymal stem cells, have shown preliminary efficacy. The Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial aimed to validate cardiopoiesis-based biotherapy in a larger heart failure cohort.
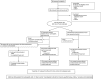
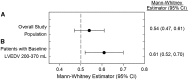
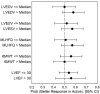
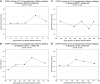
Methods and results: This multinational, randomized, double-blind, sham-controlled study was conducted in 39 hospitals. Patients with symptomatic ischaemic heart failure on guideline-directed therapy (n = 484) were screened; n = 348 underwent bone marrow harvest and mesenchymal stem cell expansion. Those achieving > 24 million mesenchymal stem cells (n = 315) were randomized to cardiopoietic cells delivered endomyocardially with a retention-enhanced catheter (n = 157) or sham procedure (n = 158). Procedures were performed as randomized in 271 patients (n = 120 cardiopoietic cells, n = 151 sham). The primary efficacy endpoint was a Finkelstein-Schoenfeld hierarchical composite (all-cause mortality, worsening heart failure, Minnesota Living with Heart Failure Questionnaire score, 6-min walk distance, left ventricular end-systolic volume, and ejection fraction) at 39 weeks. The primary outcome was neutral (Mann-Whitney estimator 0.54, 95% confidence interval [CI] 0.47-0.61 [value > 0.5 favours cell treatment], P = 0.27). Exploratory analyses suggested a benefit of cell treatment on the primary composite in patients with baseline left ventricular end-diastolic volume 200-370 mL (60% of patients) (Mann-Whitney estimator 0.61, 95% CI 0.52-0.70, P = 0.015). No difference was observed in serious adverse events. One (0.9%) cardiopoietic cell patient and 9 (5.4%) sham patients experienced aborted or sudden cardiac death.
Conclusion: The primary endpoint was neutral, with safety demonstrated across the cohort. Further evaluation of cardiopoietic cell therapy in patients with elevated end-diastolic volume is warranted.
Keywords: Cardiopoiesis; Cardiovascular disease; Disease severity; Marker; Precision medicine; Regenerative medicine; Stem cell; Target population.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Comment in
-
The quest for a successful cell-based therapeutic approach for heart failure.Eur Heart J. 2017 Mar 1;38(9):661-664. doi: 10.1093/eurheartj/ehw626. Eur Heart J. 2017. PMID: 28073861 Free PMC article. No abstract available.
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de FS, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB.. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. - PubMed
-
- Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE.. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010;56:392–406. - PMC - PubMed
-
- Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812–824. - PubMed
-
- Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ.. Cell therapy for cardiac repair–lessons from clinical trials. Nat Rev Cardiol 2014;11:232–246. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

