Retinoic acid maintains human skeletal muscle progenitor cells in an immature state
- PMID: 28025671
- PMCID: PMC11107588
- DOI: 10.1007/s00018-016-2445-1
Retinoic acid maintains human skeletal muscle progenitor cells in an immature state
Abstract
Muscle satellite cells are resistant to cytotoxic agents, and they express several genes that confer resistance to stress, thus allowing efficient dystrophic muscle regeneration after transplantation. However, once they are activated, this capacity to resist to aggressive agents is diminished resulting in massive death of transplanted cells. Although cell immaturity represents a survival advantage, the signalling pathways involved in the control of the immature state remain to be explored. Here, we show that incubation of human myoblasts with retinoic acid impairs skeletal muscle differentiation through activation of the retinoic-acid receptor family of nuclear receptor. Conversely, pharmacologic or genetic inactivation of endogenous retinoic-acid receptors improved myoblast differentiation. Retinoic acid inhibits the expression of early and late muscle differentiation markers and enhances the expression of myogenic specification genes, such as PAX7 and PAX3. These results suggest that the retinoic-acid-signalling pathway might maintain myoblasts in an undifferentiated/immature stage. To determine the relevance of these observations, we characterised the retinoic-acid-signalling pathways in freshly isolated satellite cells in mice and in siMYOD immature human myoblasts. Our analysis reveals that the immature state of muscle progenitors is correlated with high expression of several genes of the retinoic-acid-signalling pathway both in mice and in human. Taken together, our data provide evidences for an important role of the retinoic-acid-signalling pathway in the regulation of the immature state of muscle progenitors.
Keywords: Differentiation; MyoD; Myoblasts; RAR; Satellite cells.
Conflict of interest statement
The authors have declared no conflict of interest
Figures

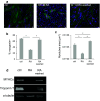
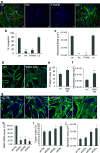
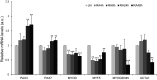
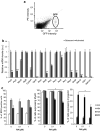
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

