Reverse transendothelial cell migration in inflammation: to help or to hinder?
- PMID: 28025672
- PMCID: PMC11107488
- DOI: 10.1007/s00018-016-2444-2
Reverse transendothelial cell migration in inflammation: to help or to hinder?
Abstract
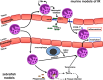
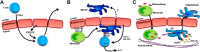
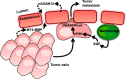
The endothelium provides a strong barrier separating circulating blood from tissue. It also provides a significant challenge for immune cells in the bloodstream to access potential sites of infection. To mount an effective immune response, leukocytes traverse the endothelial layer in a process known as transendothelial migration. Decades of work have allowed dissection of the mechanisms through which immune cells gain access into peripheral tissues, and subsequently to inflammatory foci. However, an often under-appreciated or potentially ignored question is whether transmigrated leukocytes can leave these inflammatory sites, and perhaps even return across the endothelium and re-enter circulation. Although evidence has existed to support "reverse" transendothelial migration for a number of years, it is only recently that mechanisms associated with this process have been described. Here we review the evidence that supports both reverse transendothelial migration and reverse interstitial migration within tissues, with particular emphasis on some of the more recent studies that finally hint at potential mechanisms. Additionally, we postulate the biological significance of retrograde migration, and whether it serves as an additional mechanism to limit pathology, or provides a basis for the dissemination of systemic inflammation.
Keywords: Endothelial cell; Intravasation; Monocyte; Neutrophil; Reverse interstitial migration; Reverse migration; T cell; Transmigration; rTEM.
Figures
Similar articles
-
Role of reverse transendothelial migration of neutrophils in inflammation.Biol Chem. 2016 Jun 1;397(6):497-506. doi: 10.1515/hsz-2015-0309. Biol Chem. 2016. PMID: 26872312 Review.
-
Neutrophil transendothelial migration hotspots - mechanisms and implications.J Cell Sci. 2021 Apr 1;134(7):jcs255653. doi: 10.1242/jcs.255653. J Cell Sci. 2021. PMID: 33795378 Review.
-
Pericytes support neutrophil transmigration via interleukin-8 across a porcine co-culture model of the blood-brain barrier.Brain Res. 2013 Aug 2;1524:1-11. doi: 10.1016/j.brainres.2013.05.047. Epub 2013 Jun 14. Brain Res. 2013. PMID: 23769734
-
Leukocyte migration into inflamed tissues.Immunity. 2014 Nov 20;41(5):694-707. doi: 10.1016/j.immuni.2014.10.008. Epub 2014 Nov 20. Immunity. 2014. PMID: 25517612 Review.
-
Endothelial junction regulation: a prerequisite for leukocytes crossing the vessel wall.J Innate Immun. 2013;5(4):324-35. doi: 10.1159/000348828. Epub 2013 Apr 3. J Innate Immun. 2013. PMID: 23571667 Free PMC article. Review.
Cited by
-
Monocyte recruitment and fate specification after myocardial infarction.Am J Physiol Cell Physiol. 2020 Nov 1;319(5):C797-C806. doi: 10.1152/ajpcell.00330.2020. Epub 2020 Sep 2. Am J Physiol Cell Physiol. 2020. PMID: 32877204 Free PMC article. Review.
-
Bioenergetics underlying single-cell migration on aligned nanofiber scaffolds.Am J Physiol Cell Physiol. 2020 Mar 1;318(3):C476-C485. doi: 10.1152/ajpcell.00221.2019. Epub 2019 Dec 25. Am J Physiol Cell Physiol. 2020. PMID: 31875698 Free PMC article.
-
T-Cell Adhesion in Healthy and Inflamed Skin.JID Innov. 2021 Apr 30;1(2):100014. doi: 10.1016/j.xjidi.2021.100014. eCollection 2021 Jun. JID Innov. 2021. PMID: 35024681 Free PMC article. Review.
-
Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation.Cells. 2022 Jun 15;11(12):1935. doi: 10.3390/cells11121935. Cells. 2022. PMID: 35741064 Free PMC article. Review.
-
Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea.Cell Death Dis. 2020 Aug 26;11(8):707. doi: 10.1038/s41419-020-02914-y. Cell Death Dis. 2020. PMID: 32848141 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

