Alzheimer's disease: experimental models and reality
- PMID: 28025715
- PMCID: PMC5253109
- DOI: 10.1007/s00401-016-1662-x
Alzheimer's disease: experimental models and reality
Abstract
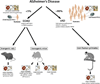
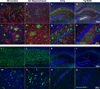
Experimental models of Alzheimer's disease (AD) are critical to gaining a better understanding of pathogenesis and to assess the potential of novel therapeutic approaches. The most commonly used experimental animal models are transgenic mice that overexpress human genes associated with familial AD (FAD) that result in the formation of amyloid plaques. However, AD is defined by the presence and interplay of both amyloid plaques and neurofibrillary tangle pathology. The track record of success in AD clinical trials thus far has been very poor. In part, this high failure rate has been related to the premature translation of highly successful results in animal models that mirror only limited aspects of AD pathology to humans. A greater understanding of the strengths and weakness of each of the various models and the use of more than one model to evaluate potential therapies would help enhance the success of therapy translation from preclinical studies to patients. In this review, we summarize the pathological features and limitations of the major experimental models of AD, including transgenic mice, transgenic rats, various physiological models of sporadic AD and in vitro human cell culture models.
Figures
References
-
- Andorfer C, Kress Y, Espinoza M, de SR, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
-
- Bales KR, Verina T, Dodel RC, Du YS, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nature Gen. 1997;17:263–264. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

