Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue
- PMID: 28025824
- PMCID: PMC5350439
- DOI: 10.1113/JP273395
Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue
Abstract
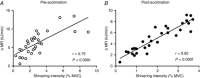
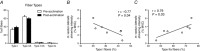
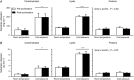
Key points: Muscle-derived thermogenesis during acute cold exposure in humans consists of a combination of cold-induced increases in skeletal muscle proton leak and shivering. Daily cold exposure results in an increase in brown adipose tissue oxidative capacity coupled with a decrease in the cold-induced skeletal muscle proton leak and shivering intensity. Improved coupling between electromyography-determined muscle activity and whole-body heat production following cold acclimation suggests a maintenance of ATPase-dependent thermogenesis and decrease in skeletal muscle ATPase independent thermogenesis. Although daily cold exposure did not change the fibre composition of the vastus lateralis, the fibre composition was a strong predictor of the shivering pattern evoked during acute cold exposure.
Abstract: We previously showed that 4 weeks of daily cold exposure in humans can increase brown adipose tissue (BAT) volume by 45% and oxidative metabolism by 182%. Surprisingly, we did not find a reciprocal reduction in shivering intensity when exposed to a mild cold (18°C). The present study aimed to determine whether changes in skeletal muscle oxidative metabolism or shivering activity could account for these unexpected findings. Nine men participated in a 4 week cold acclimation intervention (10°C water circulating in liquid-conditioned suit, 2 h day-1 , 5 days week-1 ). Shivering intensity and pattern were measured continuously during controlled cold exposure (150 min at 4 °C) before and after the acclimation. Muscle biopsies from the m. vastus lateralis were obtained to measure oxygen consumption rate and proton leak of permeabilized muscle fibres. Cold acclimation elicited a modest 21% (P < 0.05) decrease in whole-body and m. vastus lateralis shivering intensity. Furthermore, cold acclimation abolished the acute cold-induced increase in proton leak. Although daily cold exposure did not change the fibre composition of the m. vastus lateralis, fibre composition was a strong predictor of the shivering pattern evoked during acute cold. We conclude that muscle-derived thermogenesis during acute cold exposure in humans is not only limited to shivering, but also includes cold-induced increases in proton leak. The efficiency of muscle oxidative phosphorylation improves with cold acclimation, suggesting that reduced muscle thermogenesis occurs through decreased proton leak, in addition to decreased shivering intensity as BAT capacity and activity increase. These changes occur with no net difference in whole-body thermogenesis.
Keywords: cold-acclimation; energy metabolism; non-shivering thermogenesis; proton leak; shivering; uncoupling.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures



References
-
- Aguer C, Mercier J, Man CY, Metz L, Bordenave S, Lambert K, Jean E, Lantier L, Bounoua L, Brun JF, Raynaud de Mauverger E, Andreelli F, Foretz M & Kitzmann M (2010). Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia 53, 1151–1163. - PubMed
-
- Bae KA, An NY, Kwon YW, Kim C, Yoon CS, Park SC & Kim CK (2003). Muscle fibre size and capillarity in Korean diving women. Acta Physiol Scand 179, 167–172. - PubMed
-
- Bell DG, Tikuisis P & Jacobs I (1992). Relative intensity of muscular contraction during shivering. J Appl Physiol 72, 2336–2342. - PubMed
-
- Bergstrom J (1962). Muscle electrolytes in man. Scand J Clin Lab Invest Suppl 68, 7–110.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

