Dendritic cell-based immunotherapy
- PMID: 28025976
- PMCID: PMC5223236
- DOI: 10.1038/cr.2016.157
Dendritic cell-based immunotherapy
Abstract
Immunotherapy using dendritic cell (DC)-based vaccination is an approved approach for harnessing the potential of a patient's own immune system to eliminate tumor cells in metastatic hormone-refractory cancer. Overall, although many DC vaccines have been tested in the clinic and proven to be immunogenic, and in some cases associated with clinical outcome, there remains no consensus on how to manufacture DC vaccines. In this review we will discuss what has been learned thus far about human DC biology from clinical studies, and how current approaches to apply DC vaccines in the clinic could be improved to enhance anti-tumor immunity.
Figures
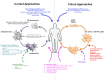
References
-
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 2012; 30:1–22. - PubMed
-
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010; 234:45–54. - PubMed
-
- Schlitzer A, Sivakamasundari V, Chen J, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol 2015; 16:718–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

