Charge-based precipitation of extracellular vesicles
- PMID: 28025988
- PMCID: PMC5065305
- DOI: 10.3892/ijmm.2016.2759
Charge-based precipitation of extracellular vesicles
Abstract
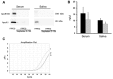
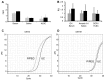
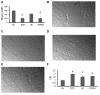
Vesicular-mediated communication between cells appears critical in many biological processes. Extracellular vesicles (EVs) released from healthy and diseased cells are involved in a network of exchange of biologically active molecules. Since EVs present in biological fluids carry the signature of the cell of origin, they are potential biomarkers for ongoing physiological or pathological processes. Despite the knowledge on EV biology accrued in recent years, techniques of EV purification remain a challenge and all the described methods have some advantages and disadvantages. In the present study, we described a method based on charge precipitation of EVs from biological fluids and from cell supernatants in comparison with the differential ultracentrifugation, which is considered the gold standard for EV purification. The analysis of ζ‑potential revealed that EVs have a negative charge that allows the interaction with a positively charged molecule, such as protamine. Protamine was shown to induce EV precipitation from serum and saliva and from cell culture media without the need for ultracentrifugation. EV resuspension was facilitated when protamine (P) precipitation was performed in the presence of PEG 35,000 Da (P/PEG precipitation). The recovery of precipitated EVs evaluated by NanoSight analysis was more efficient than that obtained by ultracentrifugation. By electron microscopy the size of EVs was similar after both methods were used, and the expression of CD63, CD9 and CD81 exosomal markers in the P/PEG‑precipitated EVs indicated an enrichment in exosomes. The RNA recovery of P/PEG‑precipitated EVs was similar to that of EVs isolated by ultracentrifugation. In addition, P/PEG‑precipitated EVs retained the biological activity in vitro as observed by the induction of wound closure by keratinocytes and of proliferation of tubular epithelial cells. In conclusion, charge-based precipitation of EVs has the merit of simplicity and avoids the requirement of expensive equipments and may be used for the efficient isolation of EVs from small biological samples.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

