Elevated intracellular Na+ concentrations in developing spinal neurons
- PMID: 28027400
- PMCID: PMC5310982
- DOI: 10.1111/jnc.13936
Elevated intracellular Na+ concentrations in developing spinal neurons
Abstract
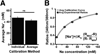
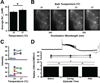
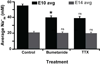
Over 25 years ago it was first reported that intracellular chloride levels (Cl-in ) were higher in developing neurons than in maturity. This finding has had significant implications for understanding the excitability of developing networks and recognizing the underlying causes of hyperexcitability associated with disease and neural injury. While there is some evidence that intracellular sodium levels (Na+in ) change during the development of non-neural cells, it has largely been assumed that Na+in is the same in developing and mature neurons. Here, using the sodium indicator SBFI, we test this idea and find that Na+in is significantly higher in embryonic spinal motoneurons and interneurons than in maturity. We find that Na+in reaches ~ 60 mM in mid-embryonic development and is then reduced to ~ 30 mM in late embryonic development. By retrogradely labeling motoneurons with SBFI we can reliably follow Na+in levels in vitro for hours. Bursts of spiking activity, and blocking voltage-gated sodium channels did not influence observed motoneuron sodium levels. On the other hand, Na+in was reduced by blocking the Na+ -K+ -2Cl- cotransporter NKCC1, and was highly sensitive to changes in external Na+ and a blocker of the Na+ /K+ ATPase. Our findings suggest that the Na+ gradient is weaker in embryonic neuronal development and strengthens in maturity in a manner similar to that of Cl- .
Keywords: SBFI; ATPase; chick embryo; ionic gradient; sodium.
© 2016 International Society for Neurochemistry.
Figures


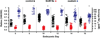
Similar articles
-
NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons.J Neurophysiol. 2009 Feb;101(2):507-18. doi: 10.1152/jn.90986.2008. Epub 2008 Nov 26. J Neurophysiol. 2009. PMID: 19036864 Free PMC article.
-
Differential contribution of the Na(+)-K(+)-2Cl(-) cotransporter NKCC1 to chloride handling in rat embryonic dorsal root ganglion neurons and motor neurons.FASEB J. 2009 Apr;23(4):1168-76. doi: 10.1096/fj.08-116012. Epub 2008 Dec 22. FASEB J. 2009. PMID: 19103648
-
Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity.J Neurophysiol. 2006 Jan;95(1):323-30. doi: 10.1152/jn.00162.2005. Epub 2005 Sep 28. J Neurophysiol. 2006. PMID: 16192339
-
Development of spinal motor networks in the chick embryo.J Exp Zool. 1992 Mar 1;261(3):261-73. doi: 10.1002/jez.1402610306. J Exp Zool. 1992. PMID: 1629659 Review.
-
Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue.J Appl Physiol (1985). 2008 Jan;104(1):288-95. doi: 10.1152/japplphysiol.01037.2007. Epub 2007 Oct 25. J Appl Physiol (1985). 2008. PMID: 17962569 Review.
Cited by
-
The subventricular zone neurogenic niche provides adult born functional neurons to repair cortical brain injuries in response to diterpenoid therapy.Stem Cell Res Ther. 2025 Jan 5;16(1):1. doi: 10.1186/s13287-024-04105-4. Stem Cell Res Ther. 2025. PMID: 39757190 Free PMC article.
-
Characterization of Active Electrode Yield for Intracortical Arrays: Awake versus Anesthesia.Micromachines (Basel). 2022 Mar 20;13(3):480. doi: 10.3390/mi13030480. Micromachines (Basel). 2022. PMID: 35334770 Free PMC article.
-
Chloride Homeostasis in Developing Motoneurons.Adv Neurobiol. 2022;28:45-61. doi: 10.1007/978-3-031-07167-6_2. Adv Neurobiol. 2022. PMID: 36066820
-
Homeostatic Recovery of Embryonic Spinal Activity Initiated by Compensatory Changes in Resting Membrane Potential.eNeuro. 2020 Jul 7;7(4):ENEURO.0526-19.2020. doi: 10.1523/ENEURO.0526-19.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32540879 Free PMC article.
-
Homeostatic Regulation of Motoneuron Properties in Development.Adv Neurobiol. 2022;28:87-107. doi: 10.1007/978-3-031-07167-6_4. Adv Neurobiol. 2022. PMID: 36066822 Review.
References
-
- Bechtold DA, Smith KJ. Sodium-mediated axonal degeneration in inflammatory demyelinating disease. J Neurol Sci. 2005;233:27–35. - PubMed
-
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. - PubMed
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

