Redox Control of Skeletal Muscle Regeneration
- PMID: 28027662
- PMCID: PMC5685069
- DOI: 10.1089/ars.2016.6782
Redox Control of Skeletal Muscle Regeneration
Abstract
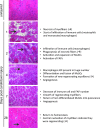
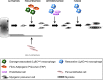
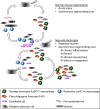
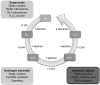
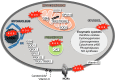
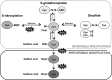
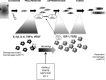
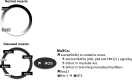
Skeletal muscle shows high plasticity in response to external demand. Moreover, adult skeletal muscle is capable of complete regeneration after injury, due to the properties of muscle stem cells (MuSCs), the satellite cells, which follow a tightly regulated myogenic program to generate both new myofibers and new MuSCs for further needs. Although reactive oxygen species (ROS) and reactive nitrogen species (RNS) have long been associated with skeletal muscle physiology, their implication in the cell and molecular processes at work during muscle regeneration is more recent. This review focuses on redox regulation during skeletal muscle regeneration. An overview of the basics of ROS/RNS and antioxidant chemistry and biology occurring in skeletal muscle is first provided. Then, the comprehensive knowledge on redox regulation of MuSCs and their surrounding cell partners (macrophages, endothelial cells) during skeletal muscle regeneration is presented in normal muscle and in specific physiological (exercise-induced muscle damage, aging) and pathological (muscular dystrophies) contexts. Recent advances in the comprehension of these processes has led to the development of therapeutic assays using antioxidant supplementation, which result in inconsistent efficiency, underlying the need for new tools that are aimed at precisely deciphering and targeting ROS networks. This review should provide an overall insight of the redox regulation of skeletal muscle regeneration while highlighting the limits of the use of nonspecific antioxidants to improve muscle function. Antioxid. Redox Signal. 27, 276-310.
Keywords: muscle stem cells; oxidative stress; skeletal muscle regeneration.
Figures







References
-
- Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, and Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J 15: 2548–2550, 2001 - PubMed
-
- Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, and Chazaud B. Autocrine and paracrine Angiopoietin 1/Tie-2 signalling promotes muscle satellite cell self-renewal. Cell Stem Cell 5: 298–309, 2009 - PMC - PubMed
-
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, and Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117: 889–901, 2007 - PMC - PubMed
-
- Al-Sawaf O, Fragoulis A, Rosen C, Keimes N, Liehn EA, Holzle F, Kan YW, Pufe T, Sonmez TT, and Wruck CJ. Nrf2 augments skeletal muscle regeneration after ischaemia-reperfusion injury. J Pathol 234: 538–547, 2014 - PubMed
-
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, and Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 14: 819–829, 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
