First-in-human safety and immunogenicity investigations of three adjuvanted reduced dose inactivated poliovirus vaccines (IPV-Al SSI) compared to full dose IPV Vaccine SSI when given as a booster vaccination to adolescents with a history of IPV vaccination at 3, 5, 12months and 5years of age
- PMID: 28027810
- PMCID: PMC5267481
- DOI: 10.1016/j.vaccine.2016.12.027
First-in-human safety and immunogenicity investigations of three adjuvanted reduced dose inactivated poliovirus vaccines (IPV-Al SSI) compared to full dose IPV Vaccine SSI when given as a booster vaccination to adolescents with a history of IPV vaccination at 3, 5, 12months and 5years of age
Abstract
Background: There is a demand of affordable IPV in the World. Statens Serum Institut (SSI) has developed three reduced dose IPV formulations adsorbed to aluminium hydroxide; 1/3 IPV-Al, 1/5 IPV-Al and 1/10 IPV-Al SSI, and now report the results of the first investigations in humans.
Methods: 240 Danish adolescents, aged 10-15years, and childhood vaccinated with IPV were booster vaccinated with 1/3 IPV-Al, 1/5 IPV-Al, 1/10 IPV-Al or IPV Vaccine SSI. The booster effects (GMTRs) of the three IPV-Al SSI were compared to IPV Vaccine SSI, and evaluated for non-inferiority.
Immunogenicity results: The pre-vaccination GMTs were similar across the groups; 926 (type 1), 969 (type 2) and 846 (type 3) in the total trial population. The GMTRs by poliovirus type and IPV formulation were: Type 1: 17.0 (1/3 IPV-Al), 13.0 (1/5 IPV-Al), 7.1 (1/10 IPV-Al) and 42.2 (IPV Vaccine SSI). Type 2: 12.5 (1/3 IPV-Al), 13.1 (1/5 IPV-Al), 7.6 (1/10 IPV-Al) and 47.8 (IPV Vaccine SSI). Type 3: 14.5 (1/3 IPV-Al), 16.2 (1/5 IPV-Al), 8.9 (1/10 IPV-Al) and 62.4 (IPV Vaccine SSI) Thus, the three IPV-Al formulations were highly immunogenic, but inferior to IPV Vaccine SSI, in this booster vaccination trial.
Safety results: No SAE and no AE of severe intensity occurred. 59.2% of the subjects reported at least one AE. Injection site pain was the most frequent AE in all groups; from 24.6% to 43.3%. Injection site redness and swelling frequencies were<5% in most and<10% in all groups. The most frequent systemic AEs were fatigue (from 8.2% to 15.0%) and headache (from 15.0% to 28.3%). Most AEs were of mild intensity. In conclusion, the three IPV-Al SSI were safe in adolescents and the booster effects were satisfactory. ClinicalTrials.gov registration number: NCT02280447.
Keywords: Affordable IPV; Aluminium hydroxide adjuvant; Dose investigation; IPV dose sparing.
Copyright © 2016. Published by Elsevier Ltd.
Figures

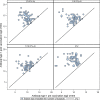
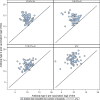

References
-
- World Health Organisation. Polio eradication & endgame strategic plan 2013-2018. WHO Press World Health Organisation, 20 Avenue Appia, Geneva, Switzerland 2013. Available from: URL: <http://www.polioeradication.org>.
-
- Okayasu H., Sutter R.W., Jafari H.S., Takane M., Aylward R.B. Affordable inactivated poliovirus vaccine: strategies and progress. J Infect Dis. 2014;1(210 Suppl 1):S459–S464. [November] - PubMed
-
- Brenzel L. What have we learned on costs and financing of routine immunization from the comprehensive multi-year plans in GAVI eligible countries? Vaccine. 2015;7(33 Suppl 1):A93–A98. [May] - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

