Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics
- PMID: 28028208
- PMCID: PMC5240713
- DOI: 10.1073/pnas.1611064114
Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics
Abstract
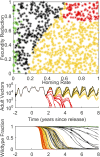
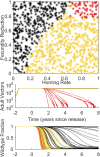
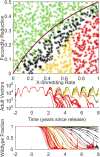
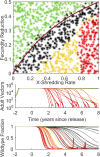
The renewed effort to eliminate malaria and permanently remove its tremendous burden highlights questions of what combination of tools would be sufficient in various settings and what new tools need to be developed. Gene drive mosquitoes constitute a promising set of tools, with multiple different possible approaches including population replacement with introduced genes limiting malaria transmission, driving-Y chromosomes to collapse a mosquito population, and gene drive disrupting a fertility gene and thereby achieving population suppression or collapse. Each of these approaches has had recent success and advances under laboratory conditions, raising the urgency for understanding how each could be deployed in the real world and the potential impacts of each. New analyses are needed as existing models of gene drive primarily focus on nonseasonal or nonspatial dynamics. We use a mechanistic, spatially explicit, stochastic, individual-based mathematical model to simulate each gene drive approach in a variety of sub-Saharan African settings. Each approach exhibits a broad region of gene construct parameter space with successful elimination of malaria transmission due to the targeted vector species. The introduction of realistic seasonality in vector population dynamics facilitates gene drive success compared with nonseasonal analyses. Spatial simulations illustrate constraints on release timing, frequency, and spatial density in the most challenging settings for construct success. Within its parameter space for success, each gene drive approach provides a tool for malaria elimination unlike anything presently available. Provided potential barriers to success are surmounted, each achieves high efficacy at reducing transmission potential and lower delivery requirements in logistically challenged settings.
Keywords: Anopheles; elimination; gene drive; malaria; mosquitoes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
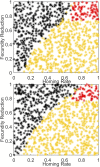

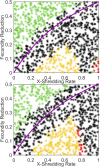
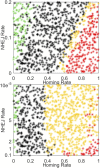
References
-
- World Health Organization . World Malaria Report 2014. World Health Organization; Geneva: 2014.
-
- Moonen B, et al. Making the decision. In: Feachem RGA, et al., editors. Shrinking the Malaria Map: A Prospectus on Malaria Elimination. The Global Health Group: UCSF Global Health Sciences; San Francisco: 2009. pp. 1–18.
-
- Alphey L, et al. Malaria control with genetically manipulated insect vectors. Science. 2002;298(5591):119–121. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

