Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes
- PMID: 28028215
- PMCID: PMC5240701
- DOI: 10.1073/pnas.1612322114
Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes
Erratum in
-
Correction to Supporting Information for Andreu-Fernández et al., Bax transmembrane domain interacts with prosurvival Bcl-2 proteins in biological membranes.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):E1574. doi: 10.1073/pnas.1701233114. Epub 2017 Feb 13. Proc Natl Acad Sci U S A. 2017. PMID: 28193890 Free PMC article. No abstract available.
Abstract
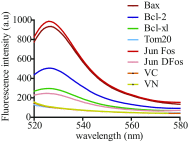
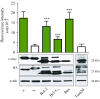
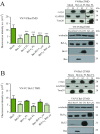
The Bcl-2 (B-cell lymphoma 2) protein Bax (Bcl-2 associated X, apoptosis regulator) can commit cells to apoptosis via outer mitochondrial membrane permeabilization. Bax activity is controlled in healthy cells by prosurvival Bcl-2 proteins. C-terminal Bax transmembrane domain interactions were implicated recently in Bax pore formation. Here, we show that the isolated transmembrane domains of Bax, Bcl-xL (B-cell lymphoma-extra large), and Bcl-2 can mediate interactions between Bax and prosurvival proteins inside the membrane in the absence of apoptotic stimuli. Bcl-2 protein transmembrane domains specifically homooligomerize and heterooligomerize in bacterial and mitochondrial membranes. Their interactions participate in the regulation of Bcl-2 proteins, thus modulating apoptotic activity. Our results suggest that interactions between the transmembrane domains of Bax and antiapoptotic Bcl-2 proteins represent a previously unappreciated level of apoptosis regulation.
Keywords: Bcl-2; apoptosis; mitochondria; oligomerization; transmembrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. - PubMed
-
- Kim H, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8(12):1348–1358. - PubMed
-
- Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17(4):525–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

