Transparent amorphous oxide semiconductors for organic electronics: Application to inverted OLEDs
- PMID: 28028243
- PMCID: PMC5240693
- DOI: 10.1073/pnas.1617186114
Transparent amorphous oxide semiconductors for organic electronics: Application to inverted OLEDs
Abstract
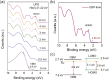
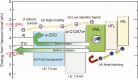
Efficient electron transfer between a cathode and an active organic layer is one key to realizing high-performance organic devices, which require electron injection/transport materials with very low work functions. We developed two wide-bandgap amorphous (a-) oxide semiconductors, a-calcium aluminate electride (a-C12A7:e) and a-zinc silicate (a-ZSO). A-ZSO exhibits a low work function of 3.5 eV and high electron mobility of 1 cm2/(V · s); furthermore, it also forms an ohmic contact with not only conventional cathode materials but also anode materials. A-C12A7:e has an exceptionally low work function of 3.0 eV and is used to enhance the electron injection property from a-ZSO to an emission layer. The inverted electron-only and organic light-emitting diode (OLED) devices fabricated with these two materials exhibit excellent performance compared with the normal type with LiF/Al. This approach provides a solution to the problem of fabricating oxide thin-film transistor-driven OLEDs with both large size and high stability.
Keywords: amorphous oxide semiconductor; electron injection; electron transport; inverted OLEDs; low work function material.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Katz HE, Huang J. Thin-film organic electronic devices. Annu Rev Mater Res. 2009;39:71–92.
-
- Gärtner C, et al. The influence of annihilation processes on the threshold current density of organic laser diodes. J Appl Phys. 2007;101(2):023107.
-
- Gong X, et al. Bulk heterojunction solar cells with large open-circuit voltage: Electron transfer with small donor-acceptor energy offset. Adv Mater. 2011;23(20):2272–2277. - PubMed
-
- Ma R. Organic Electroluminescent Displays. In: Chen J, Cranton W, Fihn M, editors. Handbook of Visual Display Technology. Vol. 2. Springer; Berlin: 2012. pp. 1210–1237.
-
- Chen CW, Lin CL, Wu CC. An effective cathode structure for inverted top-emitting organic light-emitting devices. Appl Phys Lett. 2004;85(13):2469–2471.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

