Purification of lactoperoxidase from bovine whey and investigation of kinetic parameters
- PMID: 28028529
- PMCID: PMC5156962
- DOI: 10.4103/2277-9175.192738
Purification of lactoperoxidase from bovine whey and investigation of kinetic parameters
Abstract
Background: Lactoperoxidase (LPO) is related to mammalian peroxidase family which contains a wide spectrum of biological activities. Despite the wide studies on the LPO, there is little study has been performed to simplify and shorten the procedure of enzyme purification. The aim of this project was to purify the enzyme through a simple method, and investigating enzyme kinetic parameters.
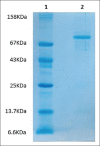
Materials and methods: LPO was purified from bovine whey through modified method of Yoshida (1990) using two steps of ammonium sulfate precipitation and ion-exchange chromatography. The purity of isolated enzyme was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
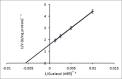
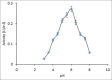
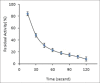
Results: The enzyme was purified 59.13-fold with a recovery of 10.26 having a specific activity of 5.78 U/mg protein and an Rz value of 0.8. The enzyme activity was measured using guaiacol as a chromogenic substrate in phosphate buffer pH 6. SDS-PAGE showed a single bond with molecular weight of 78 kDa. The purified enzyme displayed optimum activity at pH 6 in 30 mM phosphate buffer and at a temperature of 50°C, with a Km value of 178 mM and Vmax 0.63 U/ml.min for guaiacol.
Conclusion: Using only one step ion-exchange chromatography, LPO was isolated from bovine whey in high purity.
Keywords: Bovine milk; enzyme; kinetic parameters; lactoperoxidase; purification; whey.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Al-Baarri AN, Ogawa M, Hayakawa S. Application of lactoperoxidase system using bovine whey and the effect of storage condition on lactoperoxidase activity. Int J Dairy Sci. 2011;6:72–8.
-
- Kussendrager KD, van Hooijdonk AC. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br J Nutr. 2000;84(Suppl 1):S19–25. - PubMed
-
- Boots JW, Floris R. Lactoperoxidase: From catalytic mechanism to practical applications. Int Dairy J. 2006;16:1272–6.
LinkOut - more resources
Full Text Sources
Other Literature Sources

