Rapid frequency-dependent changes in free mitochondrial calcium concentration in rat cardiac myocytes
- PMID: 28028811
- PMCID: PMC5350475
- DOI: 10.1113/JP273589
Rapid frequency-dependent changes in free mitochondrial calcium concentration in rat cardiac myocytes
Abstract
Key points: Calcium ions regulate mitochondrial ATP production and contractile activity and thus play a pivotal role in matching energy supply and demand in cardiac muscle. The magnitude and kinetics of the changes in free mitochondrial calcium concentration in cardiac myocytes are largely unknown. Rapid stimulation frequency-dependent increases but relatively slow decreases in free mitochondrial calcium concentration were observed in rat cardiac myocytes. This asymmetry caused a rise in the mitochondrial calcium concentration with stimulation frequency. These results provide insight into the mechanisms of mitochondrial calcium uptake and release that are important in healthy and diseased myocardium.
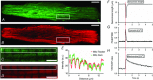
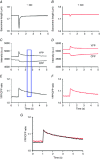
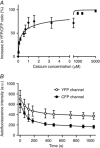
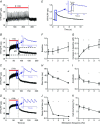
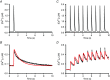
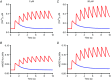
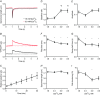
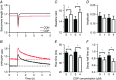
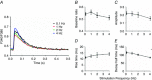
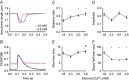
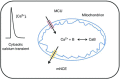
Abstract: Calcium ions regulate mitochondrial ATP production and contractile activity and thus play a pivotal role in matching energy supply and demand in cardiac muscle. Little is known about the magnitude and kinetics of the changes in free mitochondrial calcium concentration in cardiomyocytes. Using adenoviral infection, a ratiometric mitochondrially targeted Förster resonance energy transfer (FRET)-based calcium indicator (4mtD3cpv, MitoCam) was expressed in cultured adult rat cardiomyocytes and the free mitochondrial calcium concentration ([Ca2+ ]m ) was measured at different stimulation frequencies (0.1-4 Hz) and external calcium concentrations (1.8-3.6 mm) at 37°C. Cytosolic calcium concentrations were assessed under the same experimental conditions in separate experiments using Fura-4AM. The increases in [Ca2+ ]m during electrical stimulation at 0.1 Hz were rapid (rise time = 49 ± 2 ms), while the decreases in [Ca2+ ]m occurred more slowly (decay half time = 1.17 ± 0.07 s). Model calculations confirmed that this asymmetry caused the rise in [Ca2+ ]m during diastole observed at elevated stimulation frequencies. Inhibition of the mitochondrial sodium-calcium exchanger (mNCE) resulted in a rise in [Ca2+ ]m at baseline and, paradoxically, in an acceleration of Ca2+ release.
In conclusion: rapid increases in [Ca2+ ]m allow for fast adjustment of mitochondrial ATP production to increases in myocardial demand on a beat-to-beat basis and mitochondrial calcium release depends on mNCE activity and mitochondrial calcium buffering.
Keywords: calcium mitochondria; cardiac muscle; cardiomyocyte; muscle energetics.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
References
-
- Barth E, Stämmler G, Speiser B & Schaper J (1992). Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24, 669–681. - PubMed
-
- Bers DM (2002). Cardiac excitation‐contraction coupling. Nature 415, 198–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

