Mechanism-Based Modeling of Gastric Emptying Rate and Gallbladder Emptying in Response to Caloric Intake
- PMID: 28028939
- PMCID: PMC5192972
- DOI: 10.1002/psp4.12152
Mechanism-Based Modeling of Gastric Emptying Rate and Gallbladder Emptying in Response to Caloric Intake
Abstract
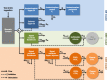
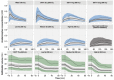
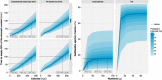
Bile acids released postprandially modify the rate and extent of absorption of lipophilic compounds. The present study aimed to predict gastric emptying (GE) rate and gallbladder emptying (GBE) patterns in response to caloric intake. A mechanism-based model for GE, cholecystokinin plasma concentrations, and GBE was developed on data from 33 patients with type 2 diabetes and 33 matched nondiabetic individuals who were administered various test drinks. A feedback action of the caloric content entering the proximal small intestine was identified for the rate of GE. The cholecystokinin concentrations were not predictive of GBE, and an alternative model linking the nutrients amount in the upper intestine to GBE was preferred. Relative to fats, the potency on GBE was 68% for proteins and 2.3% for carbohydrates. The model predictions were robust across a broad range of nutritional content and may potentially be used to predict postprandial changes in drug absorption.
© 2016 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
References
-
- Amidon, G.L. , Lennernäs, H. , Shah, V.P. & Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 12, 413–420 (1995). - PubMed
-
- Charman, W.N. , Porter, C.J. , Mithani, S. & Dressman, J.B. Physicochemical and physiological mechanisms for the effects of food on drug absorption : The role of lipids and pH. J. Pharm. Sci. 86, 269–282 (1997). - PubMed
-
- Sjögren, E. et al In vivo methods for drug absorption–Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur. J. Pharm. Sci. 57, 99–151 (2014). - PubMed
-
- Camilleri, M. Integrated upper gastrointestinal response to food intake. Gastroenterology 131, 640–58 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

