Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization
- PMID: 28029168
- PMCID: PMC5466503
- DOI: 10.1002/med.21426
Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization
Abstract
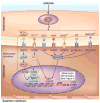
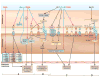
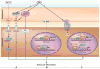
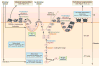
Vitiligo is the most frequent human pigmentary disorder, characterized by progressive autoimmune destruction of mature epidermal melanocytes. Of the current treatments offering partial and temporary relief, ultraviolet (UV) light is the most effective, coordinating an intricate network of keratinocyte and melanocyte factors that control numerous cellular and molecular signaling pathways. This UV-activated process is a classic example of regenerative medicine, inducing functional melanocyte stem cell populations in the hair follicle to divide, migrate, and differentiate into mature melanocytes that regenerate the epidermis through a complex process involving melanocytes and other cell lineages in the skin. Using an in-depth correlative analysis of multiple experimental and clinical data sets, we generated a modern molecular research platform that can be used as a working model for further research of vitiligo repigmentation. Our analysis emphasizes the active participation of defined molecular pathways that regulate the balance between stemness and differentiation states of melanocytes and keratinocytes: p53 and its downstream effectors controlling melanogenesis; Wnt/β-catenin with proliferative, migratory, and differentiation roles in different pigmentation systems; integrins, cadherins, tetraspanins, and metalloproteinases, with promigratory effects on melanocytes; TGF-β and its effector PAX3, which control differentiation. Our long-term goal is to design pharmacological compounds that can specifically activate melanocyte precursors in the hair follicle in order to obtain faster, better, and durable repigmentation.
Keywords: melanoblast; melanocyte stem cell; regeneration; repigmentation; vitiligo.
© 2016 Wiley Periodicals, Inc.
Figures

References
-
- Birlea SA, Spritz RA, Norris DA. Vitiligo. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 8. New York, NY: McGraw-Hill; 2012. pp. 792–803.
-
- Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: An immunohistochemical investigation. J Invest Dermatol. 1993;100:816–822. - PubMed
-
- Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, Robinson WA, Roop DR, Norris DA, Birlea SA. Narrow band ultraviolet B treatment for human vitiligo is associated with proliferation, migration, and differentiation of melanocyte precursors. J Invest Dermatol. 2015;135:2068–2076. - PMC - PubMed
-
- Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, Takigawa M, Paus R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128:1196–1206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

