E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group
- PMID: 28029303
- PMCID: PMC5455313
- DOI: 10.1200/JCO.2016.68.3300
E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group
Abstract
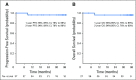
Purpose Human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) is treatment-responsive. Definitive chemoradiation results in high cure rates but causes long-term toxicity and may represent overtreatment of some patients. This phase II trial evaluated whether complete clinical response (cCR) to induction chemotherapy (IC) could select patients with HPV-associated OPSCC for reduced radiation dose as a means of sparing late sequelae. Methods Patients with HPV16 and/or p16-positive, stage III-IV OPSCC received three cycles of IC with cisplatin, paclitaxel, and cetuximab. Patients with primary-site cCR to IC received intensity-modulated radiation therapy (IMRT) 54 Gy with weekly cetuximab; those with less than cCR to IC at the primary site or nodes received 69.3 Gy and cetuximab to those regions. The primary end point was 2-year progression-free survival. Results Of the 90 patients enrolled, 80 were evaluable. Their median age was 57 years (range, 35 to 73 years), with the majority having stage T1-3N0-N2b OPSCC and a history of ≤ 10 pack-years of cigarette smoking. Three cycles of IC were delivered to 77 of the 80 patients. Fifty-six patients (70%) achieved a primary-site cCR to IC and 51 patients continued to cetuximab with IMRT 54 Gy. After median follow-up of 35.4 months, 2-year progression-free survival and overall survival rates were 80% and 94%, respectively, for patients with primary-site cCR treated with 54 Gy of radiation (n = 51); 96% and 96%, respectively, for patients with < T4, < N2c, and ≤ 10 pack-year smoking history who were treated with ≤ 54 Gy of radiation (n = 27). At 12 months, significantly fewer patients treated with a radiation dose ≤ 54 Gy had difficulty swallowing solids (40% v 89%; P = .011) or had impaired nutrition (10% v 44%; P = .025). Conclusion For IC responders, reduced-dose IMRT with concurrent cetuximab is worthy of further study in favorable-risk patients with HPV-associated OPSCC. Radiation dose reduction resulted in significantly improved swallowing and nutritional status.
Figures


Comment in
-
Is Induction Chemotherapy Needed to Select Patients for Deintensified Treatment of Human Papillomavirus-Associated Oropharyngeal Cancer?J Clin Oncol. 2017 Feb 10;35(5):479-481. doi: 10.1200/JCO.2016.71.0681. Epub 2016 Dec 28. J Clin Oncol. 2017. PMID: 28029316 No abstract available.
-
ECOG-ACRIN 1308: Commentary on a Negative Phase II Trial.J Clin Oncol. 2017 Jun 10;35(17):1969-1970. doi: 10.1200/JCO.2017.72.5085. Epub 2017 Apr 21. J Clin Oncol. 2017. PMID: 28430528 No abstract available.
-
Purpose of Induction Chemotherapy in E1308 and Importance of Patient-Reported Outcomes in Deintensification Trials.J Clin Oncol. 2017 Jun 10;35(17):1968-1969. doi: 10.1200/JCO.2017.72.2918. Epub 2017 Apr 21. J Clin Oncol. 2017. PMID: 28430529 No abstract available.
-
Reply to A. Garden et al.J Clin Oncol. 2017 Jun 10;35(17):1970-1971. doi: 10.1200/JCO.2017.72.8279. Epub 2017 Apr 21. J Clin Oncol. 2017. PMID: 28430530 No abstract available.
-
Two Sides of the Same Coin: Head and Neck Cancer Treatment De-Intensification and Intensification with Induction Chemotherapy.Int J Radiat Oncol Biol Phys. 2018 Sep 1;102(1):1-4. doi: 10.1016/j.ijrobp.2018.04.002. Epub 2018 Aug 8. Int J Radiat Oncol Biol Phys. 2018. PMID: 31200811 No abstract available.
References
-
- Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. - PubMed
-
- Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: Results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–3977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

