A New View into the Regulation of Purine Metabolism: The Purinosome
- PMID: 28029518
- PMCID: PMC5272809
- DOI: 10.1016/j.tibs.2016.09.009
A New View into the Regulation of Purine Metabolism: The Purinosome
Abstract
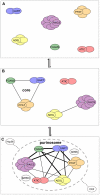
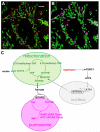
Other than serving as building blocks for DNA and RNA, purine metabolites provide a cell with the necessary energy and cofactors to promote cell survival and proliferation. A renewed interest in how purine metabolism may fuel cancer progression has uncovered a new perspective into how a cell regulates purine need. Under cellular conditions of high purine demand, the de novo purine biosynthetic enzymes cluster near mitochondria and microtubules to form dynamic multienzyme complexes referred to as 'purinosomes'. In this review, we highlight the purinosome as a novel level of metabolic organization of enzymes in cells, its consequences for regulation of purine metabolism, and the extent that purine metabolism is being targeted for the treatment of cancers.
Keywords: metabolon; purine metabolism; purinosome.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
-
- Buchanan JM, Hartman SC. Enzymic Reactions in the Synthesis of the Purines. In: Ford FF, editor. Advances in Enzymology and Related Areas of Molecular Biology. John Wiley & Sons, Inc; 1959. pp. 199–261.
-
- Greenberg GR, Jaenicke L. On the Activation of the One-Carbon Unit for the Biosynthesis of Purine Nucleotides. In: Wolstenholme GEW, O'Connor CM, editors. Ciba Foundation Symposium - Chemistry and Biology of Purines. John Wiley & Sons, Ltd; 1957. pp. 204–232.
-
- Hartman SC, Buchanan JM. Nucleic acids, purines, pyrimidines (nucleotide synthesis) Annual review of biochemistry. 1959;28:365–410. - PubMed
-
- Rudolph J, Stubbe J. Investigation of the mechanism of phosphoribosylamine transfer from glutamine phosphoribosylpyrophosphate amidotransferase to glycinamide ribonucleotide synthetase. Biochemistry. 1995;34:2241–2250. - PubMed
-
- Smith GK, et al. Characterization of the enzyme complex involving the folate-requiring enzymes of de novo purine biosynthesis. Biochemistry. 1980;19:4313–4321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

