The pseudogene DUXAP10 promotes an aggressive phenotype through binding with LSD1 and repressing LATS2 and RRAD in non small cell lung cancer
- PMID: 28029651
- PMCID: PMC5354904
- DOI: 10.18632/oncotarget.14125
The pseudogene DUXAP10 promotes an aggressive phenotype through binding with LSD1 and repressing LATS2 and RRAD in non small cell lung cancer
Abstract
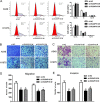
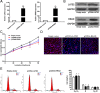
Pseudogenes have been considered as non-functional transcriptional relics of human genomic for long time. However, recent studies revealed that they play a plethora of roles in diverse physiological and pathological processes, especially in cancer, and many pseudogenes are transcribed into long noncoding RNAs and emerging as a novel class of lncRNAs. However, the biological roles and underlying mechanism of pseudogenes in the pathogenesis of non small cell lung cancer are still incompletely elucidated. This study identifies a putative oncogenic pseudogene DUXAP10 in NSCLC, which is located in 14q11.2 and 2398 nt in length. Firstly, we found that DUXAP10 was significantly up-regulated in 93 human NSCLC tissues and cell lines, and increased DUXAP10 was associated with patients poorer prognosis and short survival time. Furthermore, the loss and gain of functional studies including growth curves, migration, invasion assays and in vivo studies verify the oncogenic roles of DUXAP10 in NSCLC. Finally, the mechanistic experiments indicate that DUXAP10 could interact with Histone demethylase Lysine specific demethylase1 (LSD1) and repress tumor suppressors Large tumor suppressor 2 (LATS2) and Ras-related associated with diabetes (RRAD) transcription in NSCLC cells. Taken together, these findings demonstrate DUXAP10 exerts the oncogenic roles through binding with LSD1 and epigenetic silencing LATS2 and RRAD expression. Our investigation reveals the novel roles of pseudogene in NSCLC, which may serve as new target for NSCLC diagnosis and therapy.
Keywords: DUXAP10; LSD1; migration and invasion; proliferation; pseudogene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. - PubMed
-
- Wistuba II. Genetics of preneoplasia: lessons from lung cancer. Curr Mol Med. 2007;7:3–14. - PubMed
-
- Klebe S, Henderson DW. Facts and fiction: premalignant lesions of lung tissues. Pathology. 2013;45:305–315. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

