Spoxazomicin D and Oxachelin C, Potent Neuroprotective Carboxamides from the Appalachian Coal Fire-Associated Isolate Streptomyces sp. RM-14-6
- PMID: 28029795
- PMCID: PMC5337259
- DOI: 10.1021/acs.jnatprod.6b00948
Spoxazomicin D and Oxachelin C, Potent Neuroprotective Carboxamides from the Appalachian Coal Fire-Associated Isolate Streptomyces sp. RM-14-6
Abstract
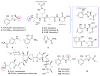
The isolation and structure elucidation of six new bacterial metabolites [spoxazomicin D (2), oxachelins B and C (4, 5), and carboxamides 6-8] and 11 previously reported bacterial metabolites (1, 3, 9-12a, and 14-18) from Streptomyces sp. RM-14-6 is reported. Structures were elucidated on the basis of comprehensive 1D and 2D NMR and mass spectrometry data analysis, along with direct comparison to synthetic standards for 2, 11, and 12a,b. Complete 2D NMR assignments for the known metabolites lenoremycin (9) and lenoremycin sodium salt (10) were also provided for the first time. Comparative analysis also provided the basis for structural revision of several previously reported putative aziridine-containing compounds [exemplified by madurastatins A1, B1, C1 (also known as MBJ-0034), and MBJ-0035] as phenol-dihydrooxazoles. Bioactivity analysis [including antibacterial, antifungal, cancer cell line cytotoxicity, unfolded protein response (UPR) modulation, and EtOH damage neuroprotection] revealed 2 and 5 as potent neuroprotectives and lenoremycin (9) and its sodium salt (10) as potent UPR modulators, highlighting new functions for phenol-oxazolines/salicylates and polyether pharmacophores.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors report competing interests. JST is a co-founder of Centrose (Madison, WI).
Figures

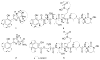


References
-
- Laatsch H. AntiBase 2014, The Natural Compound Identifier. Wiley-VCH; Weinheim: 2014.
-
- Inahashi Y, Iwatsuki M, Ishiyama A, Namatame M, Nishihara-Tsukashima A, Matsumoto A, Hirose T, Sunazuka T, Yamada H, Otoguro K, Takahashi Y, Omura S, Shiomi KJ. Antibiot. 2011;64:303–307. - PubMed
-
- Inahashi Y, Matsumoto A, Omura S, Takahashi Y. J Antibiot. 2011;64:297–302. - PubMed
-
- Sontag B, Gerlitz M, Paululat T, Rasser HF, Grun-Wollny I, Hansske FG. J Antibiot. 2006;59:659–663. - PubMed
-
- Grün-wollny I, Paululat T, Hansske FG, Sontag B. Novel Drug: Oxachelin and its Derivatives. 2005/051411. WO. 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

