Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas
- PMID: 28029918
- PMCID: PMC5508592
- DOI: 10.1056/NEJMoa1605943
Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas
Abstract
Background: Effective medical therapies are lacking for the treatment of neurofibromatosis type 1-related plexiform neurofibromas, which are characterized by elevated RAS-mitogen-activated protein kinase (MAPK) signaling.
Methods: We conducted a phase 1 trial of selumetinib (AZD6244 or ARRY-142886), an oral selective inhibitor of MAPK kinase (MEK) 1 and 2, in children who had neurofibromatosis type 1 and inoperable plexiform neurofibromas to determine the maximum tolerated dose and to evaluate plasma pharmacokinetics. Selumetinib was administered twice daily at a dose of 20 to 30 mg per square meter of body-surface area on a continuous dosing schedule (in 28-day cycles). We also tested selumetinib using a mouse model of neurofibromatosis type 1-related neurofibroma. Response to treatment (i.e., an increase or decrease from baseline in the volume of plexiform neurofibromas) was monitored by using volumetric magnetic resonance imaging analysis to measure the change in size of the plexiform neurofibroma.
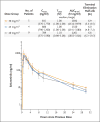
Results: A total of 24 children (median age, 10.9 years; range, 3.0 to 18.5) with a median tumor volume of 1205 ml (range, 29 to 8744) received selumetinib. Patients were able to receive selumetinib on a long-term basis; the median number of cycles was 30 (range, 6 to 56). The maximum tolerated dose was 25 mg per square meter (approximately 60% of the recommended adult dose). The most common toxic effects associated with selumetinib included acneiform rash, gastrointestinal effects, and asymptomatic creatine kinase elevation. The results of pharmacokinetic evaluations of selumetinib among the children in this trial were similar to those published for adults. Treatment with selumetinib resulted in confirmed partial responses (tumor volume decreases from baseline of ≥20%) in 17 of the 24 children (71%) and decreases from baseline in neurofibroma volume in 12 of 18 mice (67%). Disease progression (tumor volume increase from baseline of ≥20%) has not been observed to date. Anecdotal evidence of decreases in tumor-related pain, disfigurement, and functional impairment was observed.
Conclusions: Our early-phase data suggested that children with neurofibromatosis type 1 and inoperable plexiform neurofibromas benefited from long-term dose-adjusted treatment with selumetinib without having excess toxic effects. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01362803 .).
Figures


Comment in
-
Targeted therapies: Selumetinib MEKing differences in NF1.Nat Rev Clin Oncol. 2017 Mar;14(3):140. doi: 10.1038/nrclinonc.2017.6. Epub 2017 Jan 17. Nat Rev Clin Oncol. 2017. PMID: 28094260 No abstract available.
-
Selumetinib in Plexiform Neurofibromas.N Engl J Med. 2017 Mar 23;376(12):1195. doi: 10.1056/NEJMc1701029. N Engl J Med. 2017. PMID: 28332386 No abstract available.
-
Nerve sheath tumours.J Neurol. 2018 Dec;265(12):3034-3035. doi: 10.1007/s00415-018-9098-y. J Neurol. 2018. PMID: 30382390 Free PMC article. No abstract available.
References
-
- Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–51. - PubMed
-
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–43. - PubMed
-
- Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89:31–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
