Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial
- PMID: 28030470
- PMCID: PMC5359787
- DOI: 10.1097/j.pain.0000000000000814
Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial
Abstract
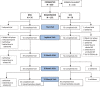
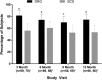
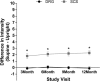
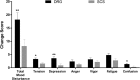
Animal and human studies indicate that electrical stimulation of dorsal root ganglion (DRG) neurons may modulate neuropathic pain signals. ACCURATE, a pivotal, prospective, multicenter, randomized comparative effectiveness trial, was conducted in 152 subjects diagnosed with complex regional pain syndrome or causalgia in the lower extremities. Subjects received neurostimulation of the DRG or dorsal column (spinal cord stimulation, SCS). The primary end point was a composite of safety and efficacy at 3 months, and subjects were assessed through 12 months for long-term outcomes and adverse events. The predefined primary composite end point of treatment success was met for subjects with a permanent implant who reported 50% or greater decrease in visual analog scale score from preimplant baseline and who did not report any stimulation-related neurological deficits. No subjects reported stimulation-related neurological deficits. The percentage of subjects receiving ≥50% pain relief and treatment success was greater in the DRG arm (81.2%) than in the SCS arm (55.7%, P < 0.001) at 3 months. Device-related and serious adverse events were not different between the 2 groups. Dorsal root ganglion stimulation also demonstrated greater improvements in quality of life and psychological disposition. Finally, subjects using DRG stimulation reported less postural variation in paresthesia (P < 0.001) and reduced extraneous stimulation in nonpainful areas (P = 0.014), indicating DRG stimulation provided more targeted therapy to painful parts of the lower extremities. As the largest prospective, randomized comparative effectiveness trial to date, the results show that DRG stimulation provided a higher rate of treatment success with less postural variation in paresthesia intensity compared to SCS.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95–110.
-
- Bean DJ, Johnson MH, Kydd RR. The outcome of complex regional pain syndrome type 1: a systematic review. J Pain 2014;15:677–90. - PubMed
-
- Blackwelder WC. Proving the null hypothesis in clinical trials. Controlled Clin Trials 1982;3:345–53. - PubMed
-
- Caspary T, Anderson KV. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nature reviews. Neuroscience 2003;4:289–97. - PubMed
-
- Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med 1994;23:129–38. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

