Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial
- PMID: 28030702
- PMCID: PMC5321662
- DOI: 10.1001/jama.2016.19425
Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial
Abstract
Importance: Zoledronic acid, a third-generation aminobisphosphonate, reduces the incidence of skeletal-related events and pain in patients with bone metastases. The optimal dosing interval for zoledronic acid is uncertain.
Objective: To determine whether zoledronic acid administered every 12 weeks is noninferior to zoledronic acid administered every 4 weeks.
Design, setting, participants: Randomized, open-label clinical trial conducted at 269 academic and community sites in the United States. Patients (n = 1822) with metastatic breast cancer, metastatic prostate cancer, or multiple myeloma who had at least 1 site of bone involvement were enrolled between May 2009 and April 2012; follow-up concluded in April 2014.
Interventions: Patients were randomized to receive zoledronic acid administered intravenously every 4 weeks (n = 911) vs every 12 weeks (n = 911) for 2 years.
Main outcomes and measures: The primary end point was the proportion of patients having at least 1 skeletal-related event (defined as clinical fracture, spinal cord compression, radiation to bone, or surgery involving bone) within 2 years after randomization and a between-group absolute difference of 7% as the noninferiority margin. Secondary end points included the proportion of patients with at least 1 skeletal-related event by disease type, pain as assessed by the Brief Pain Inventory (range, 0-10; higher scores indicate worse pain), Eastern Cooperative Oncology Group performance status (range, 0-4; higher scores indicate worse disability), incidence of osteonecrosis of the jaw, kidney dysfunction, skeletal morbidity rate (mean number of skeletal-related events per year), and, in a subset of 553 patients, suppression of bone turnover (assessed by C-terminal telopeptide levels).
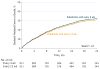
Results: Among 1822 patients who were randomized (median age, 65 years; 980 [53.8%] women; 855 with breast cancer, 689 with prostate cancer, and 278 with multiple myeloma), 795 completed the study at 2 years. A total of 260 patients (29.5%) in the zoledronic acid every 4-week dosing group and 253 patients (28.6%) in the every 12-week dosing group experienced at least 1 skeletal-related event within 2 years of randomization (risk difference of -0.3% [1-sided 95% CI, -4% to ∞]; P < .001 for noninferiority). The proportions of skeletal-related events did not differ significantly between the every 4-week dosing group vs the every 12-week dosing group for patients with breast cancer, prostate cancer, or multiple myeloma. Pain scores, performance status scores, incidence of jaw osteonecrosis, and kidney dysfunction did not differ significantly between the treatment groups. Skeletal morbidity rates were numerically identical in both groups, but bone turnover was greater (C-terminal telopeptide levels were higher) among patients who received zoledronic acid every 12 weeks.
Conclusions and relevance: Among patients with bone metastases due to breast cancer, prostate cancer, or multiple myeloma, the use of zoledronic acid every 12 weeks compared with the standard dosing interval of every 4 weeks did not result in an increased risk of skeletal events over 2 years. This longer interval may be an acceptable treatment option.
Trial registration: clinicaltrials.gov Identifier: NCT00869206.
Conflict of interest statement
Figures


Comment in
-
Zoledronic Acid Dosing Interval for Metastatic Cancer.JAMA. 2017 Apr 11;317(14):1477. doi: 10.1001/jama.2017.2558. JAMA. 2017. PMID: 28399242 No abstract available.
-
Zoledronic Acid Dosing Interval for Metastatic Cancer.JAMA. 2017 Apr 11;317(14):1477-1478. doi: 10.1001/jama.2017.2562. JAMA. 2017. PMID: 28399243 No abstract available.
-
Re: Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases: A Randomized Clinical Trial.J Urol. 2017 Sep;198(3):484. doi: 10.1016/j.juro.2017.06.009. Epub 2017 Jun 12. J Urol. 2017. PMID: 28817913 No abstract available.
-
[Can 12- instead of 4‑weekly zoledronic acid administration reduce skeletal events in patients with bone metastases?].Strahlenther Onkol. 2017 Oct;193(10):861-863. doi: 10.1007/s00066-017-1191-0. Strahlenther Onkol. 2017. PMID: 28831507 German. No abstract available.
-
Re: Effect of Longer-interval Versus Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases.Eur Urol. 2018 Feb;73(2):304. doi: 10.1016/j.eururo.2017.11.007. Epub 2017 Nov 23. Eur Urol. 2018. PMID: 29174466 No abstract available.
References
-
- Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23(15):3314–3321. - PubMed
-
- Saad F, Gleason DM, Murray R, et al. Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–1468. - PubMed
-
- Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005;(3):CD003474. - PubMed
-
- Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–8587. - PubMed
-
- Novartis Inc. [Accessed December 6, 2016];Zometa: prescribing information. 2005 Dec; http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021223s012lbl.pdf.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA052784/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- UG1 CA189870/CA/NCI NIH HHS/United States
- UG1 CA189825/CA/NCI NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- UG1 CA189861/CA/NCI NIH HHS/United States
- U10 CA045418/CA/NCI NIH HHS/United States
- U10 CA180836/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA189817/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- UG1 CA189853/CA/NCI NIH HHS/United States
- UG1 CA189819/CA/NCI NIH HHS/United States
- U10 CA074811/CA/NCI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180866/CA/NCI NIH HHS/United States
- UL1 TR001070/TR/NCATS NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U10 CA035279/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- UG1 CA189805/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA180857/CA/NCI NIH HHS/United States
- U10 CA037447/CA/NCI NIH HHS/United States
- U10 CA035272/CA/NCI NIH HHS/United States
- UG1 CA189850/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

