Tonotopic action potential tuning of maturing auditory neurons through endogenous ATP
- PMID: 28030754
- PMCID: PMC5309364
- DOI: 10.1113/JP273272
Tonotopic action potential tuning of maturing auditory neurons through endogenous ATP
Abstract
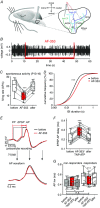
Key points: Following the genetically controlled formation of neuronal circuits, early firing activity guides the development of sensory maps in the auditory, visual and somatosensory system. However, it is not clear whether the activity of central auditory neurons is specifically regulated depending on the position within the sensory map. In the ventral cochlear nucleus, the first central station along the auditory pathway, we describe a mechanism through which paracrine ATP signalling enhances firing in a cell-specific and tonotopically-determined manner. Developmental down-regulation of P2X2/3R currents along the tonotopic axis occurs simultaneously with an increase in AMPA receptor currents, suggesting a high-to-low frequency maturation pattern. Facilitated action potential (AP) generation, measured as higher firing rate, shorter EPSP-AP delay in vivo and shorter AP latency in slice experiments, is consistent with increased synaptic efficacy caused by ATP. The long lasting change in intrinsic neuronal excitability is mediated by the heteromeric P2X2/3 receptors.
Abstract: Synaptic refinement and strengthening are activity-dependent processes that establish orderly arranged cochleotopic maps throughout the central auditory system. The maturation of auditory brainstem circuits is guided by action potentials (APs) arising from the inner hair cells in the developing cochlea. The AP firing of developing central auditory neurons can be modulated by paracrine ATP signalling, as shown for the cochlear nucleus bushy cells and principal neurons in the medial nucleus of the trapezoid body. However, it is not clear whether neuronal activity may be specifically regulated with respect to the nuclear tonotopic position (i.e. sound frequency selectivity). Using slice recordings before hearing onset and in vivo recordings with iontophoretic drug applications after hearing onset, we show that cell-specific purinergic modulation follows a precise tonotopic pattern in the ventral cochlear nucleus of developing gerbils. In high-frequency regions, ATP responsiveness diminished before hearing onset. In low-to-mid frequency regions, ATP modulation persisted after hearing onset in a subset of low-frequency bushy cells (characteristic frequency< 10 kHz). Down-regulation of P2X2/3R currents along the tonotopic axis occurs simultaneously with an increase in AMPA receptor currents, thus suggesting a high-to-low frequency maturation pattern. Facilitated AP generation, measured as higher firing frequency, shorter EPSP-AP delay in vivo, and shorter AP latency in slice experiments, is consistent with increased synaptic efficacy caused by ATP. Finally, by combining recordings and pharmacology in vivo, in slices, and in human embryonic kidney 293 cells, it was shown that the long lasting change in intrinsic neuronal excitability is mediated by the P2X2/3R.
Keywords: AP modulation; ATP release; P2X2/X3 receptor; auditory brainstem; calyceal synapses; development.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures






Comment in
-
Wake up your ears! ATP sculpts development along the auditory system.J Physiol. 2017 Feb 15;595(4):1019-1020. doi: 10.1113/JP273722. J Physiol. 2017. PMID: 28198016 Free PMC article. No abstract available.
References
-
- Abbott LF & Nelson SB (2000). Synaptic plasticity: taming the beast. Nat Neurosci 3, 1178–1183. - PubMed
-
- Anderson‐Sprecher R (1994). Model comparisons and R 2 . AmStat 48, 113–117.
-
- Aponte JE, Kotak VC & Sanes DH (1996). Decreased synaptic inhibition leads to dendritic hypertrophy prior to the onset of hearing. AudNeurosci 2, 235–240.
-
- Baqi Y, Hausmann R, Rosefort C, Rettinger J, Schmalzing G & Müller CE (2011). Discovery of potent competitive antagonists and positive modulators of the P2X2 receptor. J Med Chem 54, 817–830. - PubMed
-
- Bean BP (2007). The action potential in mammalian central neurons. Nat Rev Neurosci 8, 451–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

